Abstract
Macroscopic electric motors continue to have a large impact on almost every aspect of modern society. Consequently, the effort towards developing molecular motors1,2,3 that can be driven by electricity could not be more timely. Here we describe an electric molecular motor based on a [3]catenane4,5, in which two cyclobis(paraquat-p-phenylene)6 (CBPQT4+) rings are powered by electricity in solution to circumrotate unidirectionally around a 50-membered loop. The constitution of the loop ensures that both rings undergo highly (85%) unidirectional movement under the guidance of a flashing energy ratchet7,8, whereas the interactions between the two rings give rise to a two-dimensional potential energy surface (PES) similar to that shown by FOF1 ATP synthase9. The unidirectionality is powered by an oscillating10 voltage11,12 or external modulation of the redox potential13. Initially, we focused our attention on the homologous [2]catenane, only to find that the kinetic asymmetry was insufficient to support unidirectional movement of the sole ring. Accordingly, we incorporated a second CBPQT4+ ring to provide further symmetry breaking by interactions between the two mobile rings. This demonstration of electrically driven continual circumrotatory motion of two rings around a loop in a [3]catenane is free from the production of waste products and represents an important step towards surface-bound14 electric molecular motors.
Similar content being viewed by others
Main
During the past 40 years, the design and synthesis15,16 of artificial molecular machines have fostered the promise17,18,19 of a technological revolution similar in magnitude to that arising from the development of macroscopic motors. To convert energy from an external source into unidirectional movement on a molecular scale20,21, several artificial molecular machines, including3,22,23 rotaxane-based linear motors5,8,24,25,26,27 and catenane-based rotary motors4,5,28,29, have been designed, synthesized and shown to work in the presence of light4,24,30,31,32 and chemical fuels5,25,29,33,34. Although it has been demonstrated35,36,37 that single-molecule motors can be powered using tunnelling currents under ultrahigh vacuum on surfaces, examples of electrically powered catenane rotary motors operating in solution are not known to the best of our knowledge. Here we report the design, synthesis and operation of a redox-driven rotary motor based on a [3]catenane in which two rings can be powered by electricity to rotate unidirectionally around a loop.
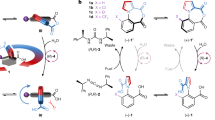
The [3]catenane molecular motor [3]CMM comprises (Fig. 1a) two CBPQT4+ (ref. 6) rings encircling a 50-membered loop. A bis(4-methylenephenyl)methane (BPM) unit separates two viologen (V2+) units, which are preordained, on reduction to their V+• reduced radical cationic states, to serve as recognition sites38 for reduced CBPQT2(+•) rings. The remainder of the loop is composed of a chain containing 11 methylene groups and one oxygen atom, intercepted along its length by an isopropylphenylene (IPP) steric barrier, a triazole (T) ring, which is generated during the final ring closure of the loop to give the [3]catenane, and a 2,6-dimethylpyridinium (PY+) Coulombic barrier. As a consequence of the redox properties of the V2+/+• units in the loop and the two CBPQT4+/2(+•) rings, the oxidized state [3]CMM13+ can be converted (Fig. 1b) into a reduced state [3]CMM7+6•, accompanied by movement of the two CBPQT2(+•) rings, so as to encircle the V+• units in the loop by virtue of radical-pairing interactions39. This design received its inspiration from previous investigations12,25,40,41 on the redox-driven rotaxane-based molecular pumps, in which a pumping cassette26,42,43, comprising PY+–V2+–IPP, serves as an active one-way gate to transport a CBPQT4+ ring from the bulk solution onto a collecting chain after every redox cycle. In comparison with these linear rotaxane-based pumps, catenane-based motors are expected to undergo continuous unidirectional circumrotation of one ring about the other, as long as there is a power source. The obvious design in which the two ends of a rotaxane-based pump are linked to form a [2]catenane gives rise to a constitution in which there is no probe of unidirectionality. Consequently, we developed the loop and the [3]catenane to investigate the interactions between and the unidirectionality of the two mobile rings.
a, Graphical representations with key structural fragments for the oxidized state of the [3]catenane molecular motor [3]CMM13+. The cyclobis(paraquat-p-phenylene) rings, the bisradical dicationic states of cyclobis(paraquat-p-phenylene), the viologens, the radical cationic states of the viologens, the bis(4-methylenephenyl)methane, the isopropylphenylene, the triazole and the 2,6-dimethypyridinium units are labelled as CBPQT4+, CBPQT2(+•), V2+, V+•, BPM, IPP, T and PY+, respectively. b, Graphical representations for the reduced state of the [3]catenane molecular motor [3]CMM7+6• with key superstructural formulas showing the radical-pairing interactions between the CBPQT2(+•) rings and the V+• units. Positive charges are balanced by PF6− counterions, which are omitted for the sake of clarity. c, The redox operation of [3]CMM13+/7+6• demonstrating the unidirectional rotary motion of the two CBPQT4+/CBPQT2+• rings. In state I, [CBPQT-A]4+ and [CBPQT-B]4+ are positioned around the T and BPM units, respectively. Reduction of the V2+ units and the CBPQT4+ rings by the injection (step 1) of six electrons in total triggers both rings to undergo a clockwise rotation, leading to the formation (state II) of the reduced state [3]CMM7+6•. Subsequent oxidation by the removal (step 2) of six electrons restores the Coulombic repulsion between the two rings and the loop, obliging [CBPQT-B]4+ to thread over (state III) the steric barrier (IPP) under thermal activation and eventually encircle T, whereas [CBPQT-A]4+ finds itself threaded around BPM, thus completing a 180° positional exchange between the two rings shown in state I′. A second redox cycle (steps 3 and 4) resets the system back to state I after the accomplishment of another 180° positional exchange between the two rings.
Initially, we studied (Supplementary Scheme 5) the homologous [2]catenane [2]C9+, in which only one CBPQT4+ ring encircles the BPM unit on the loop. No directional motion was observed (Supplementary Fig. 35) in the [2]catenane. Instead, the single CBPQT4+/2(+•) ring switches between one of the V2+/+• units and the BPM unit during a redox cycle. To gain further insight into the switching behaviour of the [2]catenane, quantum mechanical calculations were undertaken (Supplementary Information Section 6.1). Several features were shown (Extended Data Figs. 1 and 2) in the calculated PESs of the oxidized and reduced forms of the [2]catenane. (1) The energy of the electrostatic barriers arising from the PY+ and V2+/+• units, as well as the interaction energies between the CBPQT4+/2(+•) ring and the V2+/+• units, can be modulated simultaneously by switching between the oxidized and reduced states of the [2]catenane. (2) The height of the steric barrier imposed by the IPP unit is independent of the redox state and is large enough (ΔE > 25 kcal mol−1) to preclude the passage of the CBPQT4+/2(+•) ring over the IPP unit. (3) An energy well, close to the centre of the BPM unit, traps the CBPQT4+ ring under oxidizing conditions, thereby preventing directional movement on relevant timescales.
These results motivated our investigation of the [3]catenane. When two CBPQT4+ rings encircle the loop under oxidizing conditions, one ring occupies the well provided by the BPM unit, thus requiring the passage of the other ring over the IPP unit. This combination of two mechanically interlocked44 CBPQT4+/2(+•) rings, a switchable barrier, associated with the PY+ unit, two switchable viologen (V2+/+•) recognition sites and a steric barrier (IPP) is key to inducing unidirectional motion (Fig. 1c) of the two rings with respect to the loop. Let us consider the mechanism of operation (Fig. 1c). To aid the discussion, the CBPQT4+ rings are given the descriptors [CBPQT-A]4+ and [CBPQT-B]4+ (Fig. 1c, I). At the outset, on account of repulsive Coulombic forces, [CBPQT-A]4+ resides around the T unit, whereas [CBPQT-B]4+ encircles the BPM unit. On reduction, six electrons are injected (Fig. 1c, step 1) into the [3]catenane, resulting in both CBPQT4+ rings being reduced to CBPQT2(+•) and the V2+ units to V+•. The Coulombic interactions are decreased,allowing the attractive radical–radical interactions to dominate (Fig. 1c, II). As indicated (Extended Data Fig. 3) by the PESs of the reduced [3]catenane, the relative energy barrier for a CBPQT2(+•) ring to traverse the PY+ unit is much lower than the barrier for the same ring to traverse the IPP unit. It follows that [CBPQT-A]2(+•) passes over the PY+ unit to thread on to V+• in a clockwise direction and [CBPQT-B]2(+•) moves concomitantly to occupy the V+• unit that neighbours the IPP unit. In this overall reduced state, the stable [3]CMM7+6• form is the one in which both CBPQT2(+•) rings encircle38 the V+• binding sites. Subsequent oxidation (Fig. 1c, step 2) leads (Extended Data Fig. 4) to a biased Brownian motion7,45 (Fig. 1c, III) to install the [CBPQT-A]4+ ring on the BPM unit and the [CBPQT-B]4+ ring on the T unit. Overall, one redox cycle triggers the positional exchange (Fig. 1c, I′) between the two CBPQT4+ rings and completes a 180° unidirectional rotation. A subsequent redox cycle (Fig. 1c, steps 3 and 4) brings the rings back to their initial starting positions and finishes a full 360° clockwise circumrotation around the loop. Steps 3 and 4 are identical to steps 1 and 2, respectively, but with the roles of the [CBPQT-A]4+/2(+•) and [CBPQT-B]4+/2(+•) rings reversed. The use of the term ‘clockwise’ means that each CBPQT4+ ring visits (Extended Data Fig. 5) the substituents on the loop in the order T/PY+/IPP, rather than the other way around.
The [3]catenane was synthesized (Supplementary Scheme 6) by using radical templation41 leading, in the first instance, to the formation of an intermediate pseudo[3]rotaxane before the final loop closure. The oxidized state [3]CMM13+, which was isolated as its PF6− salt, was fully characterized (Fig. 2a) by 1H nuclear magnetic resonance (NMR) spectroscopy. The positions of each CBPQT4+ ring on the loop were identified by changes in the chemical shifts of proton resonances on the BPM unit (H-13 and H-14) and on the triazole ring (H-28) as a result of the strong shielding by the encircling CBPQT4+ rings. The reduced state [3]CMM7+6• of the [3]catenane, which was produced on the addition of 6.0 molar equivalents of cobaltocene26 (Cp2Co), or an excess of Zn dust25, into an MeCN solution of [3]CMM•13PF6 was accompanied by an instantaneous change from colourless to dark purple. The formation of [3]CMM7+6• can also be followed (Supplementary Fig. 48) by visible/near infrared (Vis/NIR) spectroscopy with a broad absorption band centred on 1,122 nm, which is characteristic38 of trisradical interactions. The solid-state structure of [3]CMM7+6• was characterized by X-ray crystallography (Fig. 2b and Supplementary Information Section 7), which confirms the presence of the radical-pairing interactions (Supplementary Fig. 44) between the reduced CBPQT2(+•) rings and the V+• units. On addition of NOPF6, reoxidation of [3]CMM7+6•, which occurs within seconds, is accompanied by the MeCN solution reverting back to being colourless. The reversible switching between the oxidized and reduced states of the [3]catenane was also investigated (Extended Data Fig. 6 and Supplementary Information Section 8) by cyclic voltammetry, which was carried out on [3]CMM•13PF6 in an MeCN solution. These investigations demonstrated that these two stable states of the [3]catenane can be interconverted using either chemical or electrochemical stimuli.
a, Structural formula for the oxidized state [3]CMM13+ with an optimized quantum mechanical model structure (M06-2X/6-31G* basis set, in tubular with superimposed space-filling representation) and the 1H NMR spectrum (500 MHz, CD3COCD3, 298 K), in which all the proton assignments are labelled. b, X-ray single-crystal structure of the reduced state [3]CMM7+6• depicted by tubular with superimposed space-filling representations. Solvent molecules, counterions and hydrogen atoms are omitted for the sake of clarity. c, Vis/NIR spectra of the reduced state [3]CMM7+6• (purple) and the oxidized state [3]CMM13+ (blue) during the electrically driven operation of the molecular motor. Conditions: [3]CMM (30 μM), MeCN solution with TBAPF6 (0.1 M) as the supporting electrolyte, reduction potential −0.5 V (versus Ag/AgCl) for 10 min, oxidation potential +0.7 V (versus Ag/AgCl) for 15 min. Insets are photographs of the two solutions in the oxidized (colourless) and reduced (purple) states. d, Absorption intensities of [3]CMM7+6• (purple) and [3]CMM13+ (blue) at 1,122 nm, showing the reversible switching between the two redox states during each cycle. Abs, absorption; ppm, parts per million.
With the reversible redox switching of the [3]catenane demonstrated, we turned our attention to using electricity to power the molecular motor by controlled potential electrolysis11,26,41 (CPE). The electrically driven operation (Supplementary Information Section 10) of the molecular motor was accomplished in an electrochemical cell (Extended Data Fig. 7 and Supplementary Video 1) by alternating between two constant potentials (−0.5 V for reduction and +0.7 V for oxidation). The Vis/NIR spectrum of the reduced [3]catenane (30 μM) in MeCN was found to be in excellent agreement (Supplementary Fig. 49) with that obtained from the chemically driven operation with the same concentration (30 μM), confirming the validity of the CPE protocol. We repeated this protocol on the [3]catenane. The whole process was monitored (Fig. 2c) by Vis/NIR spectroscopy. The change in absorbance at 1,122 nm demonstrated (Fig. 2d) that the continuous operation of the motor can be repeated at least five times without any substantial loss in reversibility. The 1H NMR spectrum (Supplementary Fig. 51) of the final oxidized motor molecule demonstrated recovery of more than 95% of the [3]catenane with negligible degradation, an observation that is commensurate with robust and reliable operation.
By simply accepting the present design of the [3]catenane, it is not possible to investigate the unidirectionality of the motor by determining the positions of the components after one 180° circumrotation on account of the fact that the two CBPQT4+ rings are constitutionally identical. To address this conundrum, [D16]-CBPQT4+ (Supplementary Scheme 7), in which the p-xylylene units are deuterium-labelled, was introduced (Extended Data Fig. 8 and Supplementary Scheme 9) during the synthesis of a deuterium-labelled [3]catenane [Dn]-[3]CMM13+. As the CBPQT2(+•) ring does not pass over the IPP unit under reducing conditions, there is only one gate (PY+ unit) for the CBPQT2(+•) rings threading to form the intermediate pseudo[3]rotaxane. Therefore, the distribution of undeuterated CBPQT4+ rings on the T and BPM units can be influenced by sequentially adding [D16]-CBPQT2(+•) and CBPQT2(+•). The [3]catenane [Dn]-[3]CMM13+ was obtained as a mixture of four isotopologues, including [D0]-[3]CMM13+ and [D32]-[3]CMM13+, in addition to two co-constitutional isomers of [D16]-[3]CMM13+. Their presence was confirmed by high-resolution electrospray ionization mass spectrometry (Supplementary Fig. 55) and 1H NMR spectroscopy (Supplementary Fig. 56). According to the integration of probe resonances for the H-phen′ and H-phen″ in the 1H NMR spectra, the ratio of the undeuterated CBPQT4+ rings on the T and BPM units was found to be biased (Supplementary Figs. 56–59), allowing the system as a whole to be monitored. The deuterated motor [Dn]-[3]CMM13+ was subjected to CPE operation to test for the unidirectionality of circumrotation driven by electricity. The unidirectional circumrotation of the rings causes (Fig. 3) co-constitutional exchanges after one redox cycle to be quantified (Extended Data Fig. 9) on the basis of different ratios of integrated values of proton resonances (H-phen′ and H-phen″) in the 1H NMR spectrum. From the 1H NMR integrations (Fig. 3), the directionality of the molecular motor was calculated (Extended Data Fig. 10 and Supplementary Information Section 12) to be 85%, that is, 85% of the molecular motors complete a 180° unidirectional (clockwise) rotation in one redox cycle. Several chemically driven operations of the molecular motor were also performed (Supplementary Figs. 58–61), each providing comparable unidirectionalities of about 85% after one redox cycle.
Top: graphical representation of the positional exchange of the deuterium-labelled CBPQT4+ and CBPQT4+ rings on the loop after one redox cycle. Bottom: partial 1H NMR (600 MHz, CD3COCD3, 298 K) spectra of [Dn]-[3]CMM•13PF6 with proton assignments before (left) and after (right) one electrically driven redox cycle. Numbers under peaks indicate relative integrals. ppm, parts per million.
A metastable state is observed (Fig. 4a, Supplementary Section 13 and Supplementary Fig. 66) during the oxidation process, demonstrating the unidirectional movement of both rings around the loop in the [3]catenane. Comparison (Supplementary Fig. 65) between the 1H NMR spectrum of [3]CMM•13PF6 and that of the metastable state shows the locations on the loop of two rings. Large upfield shifts are observed (Supplementary Fig. 65) for the resonances associated with the protons belonging to the BPM (H-12*) and the IPP (H-22*) units, whereas resonances for protons H-14* and H-15* are shifted downfield, indicating (Fig. 4a) that one of the two CBPQT4+ rings moves away from the V2+ unit and is located asymmetrically on the BPM unit on account of net Coulombic repulsion, whereas the other ring is poised to mount the IPP steric barrier. The positions of the two rings on the loop indicate that the circumrotation towards the metastable state is in the clockwise direction. The subsequent relaxation by clockwise rotation (Extended Data Fig. 10) to the stable oxidized state is favoured. By contrast, the counterclockwise motion going from the metastable state III to the stable oxidized state I would require both of the CBPQT4+ rings to pass over the V2+ units, which is kinetically much less likely. The appearance of the metastable state and subsequent thermally activated relaxation to the stable oxidized [3]catenane was monitored (Fig. 4a and Supplementary Fig. 76) by 1H NMR spectroscopy at 298 K in CD3CN. Kinetic analysis (Fig. 4b and Supplementary Figs. 77 and 78) shows that this co-conformational rearrangement follows first-order kinetics at 298 K with an average rate constant k of (8.6 ± 0.4) × 10−4 s−1, corresponding to an energy of activation (ΔG‡) of 21.6 kcal mol−1. These values are in good agreement with the calculated (Extended Data Fig. 4) value (ΔE‡ = 20.1 kcal mol−1). The nearly identical rate constants (Supplementary Figs. 77 and 78) associated with relaxation of the metastable state of each ring suggests that, on oxidation, the two rings undergo unidirectional circumrotation (Supplementary Video 2) on account of the electrostatic interactions arising from the nanoconfinement46 present in the mechanically interlocked [3]catenane. This mechanism is different from previously reported4,5 [3]catenane-based molecular motors in that our electric molecular motor is driven by a single stimulus, namely the continuous oscillation of redox potential, that is, an applied direct current voltage.
a, Top, graphical representation and structural formula of the metastable state with an optimized quantum mechanical model structure (M06-2X/6-31G* basis set, in tubular with superimposed space-filling representation). Bottom, partial 1H NMR (600 MHz, CD3CN, 298 K) spectra of [3]CMM•13PF6 measured over time (0–60 min) immediately after a cycle of reduction (Cp2Co) and reoxidation (NOPF6), with proton assignments labelled at the top and bottom of the spectra. The proton resonances attributable to the metastable state are labelled with an asterisk. b, Top, thermal relaxation associated with the co-conformational rearrangement from the metastable state to the reoxidized state. The activation energy barrier ΔG‡ of 21.6 kcal mol−1 was determined using the Eyring equation (\(k=\frac{{k}_{{\rm{B}}}T}{h}{{\rm{e}}}^{\frac{{-\Delta G}^{\ddagger }}{RT}}\)), in which k is the reaction rate constant, T is the absolute temperature, R is the gas constant, kB is the Boltzmann constant and h is the Planck constant. Bottom, plot of the changes in the normalized integral of protons on the BPM (H-12* and H-14) and IPP (H-22* and H-25) units with time at 298 K during the transformation from the metastable to the reoxidized state, as well as the fitting curves of these data according to the first-order kinetic model. ppm, parts per million.
We often think hierarchically about components of macroscopic machines, in which one component serves to drive another. This kind of hierarchy is impossible in molecular machines, in which movements of components obey the principle of microscopic reversibility. By contrast, the emergent interactions between the two rings in the electric molecular motor that allow for directional motion are reciprocal, but in conjunction with the kinetic asymmetry introduced into the structure of the molecule, they provide a mechanism by which time-symmetric switching between reducing and oxidizing conditions can drive unidirectional rotation. It may well turn out that these noncovalent interactions under nanoconfinement play the role of what is described as ‘gearing’ in macroscopic machines.
This electrically driven molecular motor exhibits several attractive features that are expected to play an important role in the subsequent development of artificial molecular machines. The [3]catenane can be synthesized in only four steps based on the previously described protocols40,41. Moreover, no covalent bonds need to be broken during the unidirectional circumrotation of the two rings. The timescale for the completion of two redox cycles involving the unidirectional circumrotation of the two small rings through 360° with respect to the loop is only a few minutes. Furthermore, the co-constitution of the [3]catenane is compatible with attachment to the surface of an electrode by chemical modification of one of the two small rings, allowing spatially directed rotation with respect to a fixed frame of reference, thereby transducing electrical into mechanical energy at a surface.
The energy ratchet mechanism, on which this electric molecular motor is based, is powered by redox control of the two-dimensional potential energy landscape of the [3]catenane. The energy landscape is sculpted by the design of the constitution of the loop such that externally applied oscillation of the redox potential provides the energy required to harness Brownian motion of the molecular system to achieve unidirectionality. Our design represents a chemical rather than mechanical engineering approach to the development of electric molecular motors47.
Methods
Quantum mechanical calculations
To explain the working mechanism of the electric molecular motor, quantum mechanical calculations were performed at the level of density functional theory to study the PESs of the [2]catenanes [2]C9+/5+4• and the [3]catenanes [3]CMM13+/7+6•. In these calculations, the geometries were optimized in the Poisson–Boltzmann solvation model48 at the level of the M06-2X/6-31G* basis set (ref. 49) with Jaguar v.10.6 (ref. 50). Because of the complexity of the systems, all counterions were replaced by an implicit continuous dielectric solvent. To compensate for the effect of counterions, parameters resulting in a stronger solvation effect were necessary. The new solvation parameters were obtained (Supplementary Fig. 36) by fitting the experimentally measured barrier height of the CBPQT4+ dethreading from a model pseudorotaxane. The solvation parameters ɛ = 75 and R0 = 1.4 Å were chosen for the calculations performed on [2]C9+/5+4• and [3]CMM13+/7+6•.
PESs of [2]catenane
The PESs of [2]C9+/5+4• were studied (Extended Data Figs. 1 and 2) by scanning the z coordinate from the centre of CBPQT4+/2(+•), which is defined as the average position of four methylene carbon atoms of CBPQT4+/2(+•), passing over the atoms—labelled as positions 0 to 50—on the loop. The PESs are periodic because of the cyclic nature of the loop.
For the oxidized state [2]C9+, the PES (Extended Data Fig. 1 and Supplementary Table 1) reaches a maximum (position 31) between the positively charged V2+ and PY+ units, indicating strong electrostatic repulsion between the CBPQT4+ ring and the V2+/PY+ units on the loop. The other barrier (position 2) is provided by the IPP unit because of the bulky isopropyl group. Whereas the PES shows a minimum (position 0) positioned around the T unit, the other energy well (position 18) is close to the centre of the BPM unit because of donor–acceptor and van der Waals interactions between the CBPQT4+ ring and the BPM unit. The energy barriers for the CBPQT4+ ring encircling the BPM unit passing over the bulky IPP and the positively charged PY+ units are 25.5 and 46.7 kcal mol−1, respectively.
For the reduced state [2]C5+4•, the PES (Extended Data Fig. 2a) reaches a maximum (42.2 kcal mol−1) when the CBPQT2(+•) ring passes (position 2) over the bulky IPP unit. All three wells (Extended Data Fig. 2a) result from favourable radical-pairing interactions between the CBPQT2(+•) ring and the V+• units: the first (position 10) and the third (position 27) wells correspond to the CBPQT2(+•) ring encircling the two V+• units (Extended Data Fig. 2d), respectively, whereas the second well (position 21) has a compacted conformation (Extended Data Fig. 2d) because of the radical-pairing interactions between the V+• units and the CBPQT2(+•) ring, which are tilted with respect to each other.
PESs of [3]catenane
To describe the movement of the two CBPQT4+/2(+•) rings in [3]CMM13+/7+6• around the loop, a two-dimensional map was constructed (Extended Data Fig. 5), in which the x and y axes represent the positions of the CBPQT4+/2(+•) rings on the loop. Note that the map is periodic in both dimensions. To simplify the calculations, one of the two CBPQT4+/2(+•) rings (ring A or ring B) was moved to its next position, and then the second ring was allowed to relax to its local minimum. The PESs of [3]CMM13+/7+6• on the two-dimensional map were calculated by scanning the z coordinate from the centre of one CBPQT4+/2(+•) ring passing over the labelled atoms on the loop while letting every other degree of freedom, including the position of the other CBPQT4+/2(+•) ring, relax to the local minimum. Eight hypothetical paths were calculated (Supplementary Tables 3–10) for redox switching in the [3]catenanes [3]CMM13+/7+6•, which are identified in Extended Data Figs. 3b and 4b. In the calculations, it is assumed that all of the reductions and oxidations occur rapidly relative to the ring motion.
In the case of the reduction process (Extended Data Fig. 3), although all four paths experience a decrease in energy at the beginning, path R3 requires passage over the lowest barrier. In path R3, the CBPQT2(+•) ring B was moved first of all to the bottom V+• unit, followed by moving the CBPQT2(+•) ring A to pass (Extended Data Fig. 3e) over the PY+ unit to reach the end point II. In comparison, both paths R2 and R4 require the CBPQT2(+•) ring A to pass (Extended Data Fig. 3f) over the bulky IPP unit from point I to II′. As a result, the path-determining energy difference along path R4 is 4.7 kcal mol−1 higher than that along path R3. These results indicate that, under reducing conditions, the CBPQT2(+•) rings (1 and 2) strongly prefer to move from point I to II.
The X-ray single-crystal structure (Fig. 2b) of the reduced [3]catenane [3]CMM7+6• clearly shows that two CBPQT2(+•) rings encircle the two V+• units in the loop. To study the unidirectional movement of the CBPQT4+ rings under oxidizing condition, point II was set to be the starting point. The single-crystal structure was also used as the initial structure for optimizing the geometry of the [3]catenane [3]CMM13+. On oxidation, the two CBPQT4+ rings move away from the V2+ units. The question is, which direction is the more favourable one?
Four paths that all start at point II in two different directions were examined (Extended Data Fig. 4). Among the four paths, path O1 has the lowest energy barrier for the CBPQT4+ rings moving towards the end point I′, in which two CBPQT4+ rings encircle the T and BPM units. In path O1, the CBPQT4+ ring B was first moved to the BPM unit, followed by moving the CBPQT4+ ring A to the T unit, so as to lower the overall energy. By comparison, in path O2, the CBPQT4+ ring A was first moved to pass over the PY+ unit to its final location at the T unit, followed by moving the CBPQT4+ ring A to the BPM unit. The path-determining energy difference along path O2 is 8.1 kcal mol−1 higher than that along path O1. Therefore, it is far more favourable for the two CBPQT4+ rings to move from point II to point I′.
In conclusion, the quantum mechanical calculations predict unidirectional movement from point I to point II to point I′ during the redox cycle of the [3]catenanes [3]CMM13+/7+6•, which is consistent with the experimental result.
Cyclic voltammetry
To gain a better understanding of the electron-transfer processes during the redox cycle undergone by the [3]catenane [3]CMM13+, variable scan-rate cyclic voltammetry experiments (Extended Data Fig. 6) were performed.
The cyclic voltammetry profile shows (Extended Data Fig. 6) three reduction peaks with potentials at −0.08 V, −0.15 V and −0.25 V at a low scan rate (0.02 V s−1), corresponding to reduction associated with radical formation, starting from [3]CMM13+ and leading to the production of [3]CMM7+6•. The first two reduction peaks (−0.08 V and −0.15 V) account for the stepwise formation of viologen radical pairs as a consequence of the different chemical environments experienced by the two CBPQT4+ rings in the [3]catenane [3]CMM13+. The first reduction peak at −0.08 V can be assigned to the reduction of one of the V2+ units in the loop and one of the two V2+ units in the CBPQT4+ ring encircling the BPM unit, resulting in a decrease in Coulombic repulsion, while establishing stabilizing radical-pairing interactions between the mechanically interlocked components. The following reduction peak at −0.15 V can be attributed to the reduction of the other V2+ unit in the loop and one of the two V2+ units in the other CBPQT4+ ring encircling the T unit. The third reduction peak observed at −0.25 V, corresponding to two simultaneous one-electron reductions, accounts for the further reduction of both CBPQT2+(•+) monoradical trication rings to their diradical dicationic states CBPQT2(•+). The oxidation of the radical state [3]CMM7+6• back to [3]CMM13+ occurs in two steps at −0.16 V and +0.08 V. The first oxidation peak at −0.16 V can be assigned to two one-electron oxidations of the two unpaired V+• units in the two CBPQT(2+•) rings (CBPQT2(+•) → CBPQT2+(+•)), resulting in much weaker binding interactions and increased Coulombic repulsion between the components (V+• and CBPQT2+(+•)), that is, [3]CMM7+6• is oxidized to [3]CMM5+4(+•). It is followed by four simultaneous one-electron oxidations, namely two V+• units in the two CBPQT2+(•+) rings and two V•+ units in the loop are oxidized at the same oxidation potential (+0.08 V), resulting in the formation of the fully oxidized state [3]CMM13+. These observations are consistent with previously published results51.
The reduction of the radical state [3]CMM7+6• to its neutral (viologen) form [3]CMM+ involves (Supplementary Fig. 46) three sequential two-electron reversible processes. The first and less negative one (−0.77 V, peak potential) can be assigned to the reduction of the two unpaired V+• units in the two CBPQT2(+•) rings, leading to the formation of [3]CMM+4(+•), which is stabilized52 by both radical-pairing and donor–acceptor interactions. The following two-electron process accounts for the reduction of the other two V+• units in the two CBPQT (+•) rings. Finally, the reduction of the remaining two V+• units in the loop occurs at −1.01 V, reflecting the presence of mechanical bonding and the nanoconfined geometry of the [3]catenane.
As the scan rate is increased to 2.0 V s−1, only one reduction peak for the radical state [3]CMM7+6• is observed, indicating that the electron-transfer process is much faster than ring movement at this fast scan rate. This observation suggests that, under the experimental conditions used during the operation of the [3]catenane motor, the reduction to the radical state [3]CMM7+6• and the reoxidation to the fully oxidized state [3]CMM13+ is completed fully and very rapidly.
Electrically driven operation of [3]CMM
Electrically driven operation of the [3]catenane molecular motor [3]CMM was conducted in the N2-filled glovebox. An MeCN (38 ml, 0.1 M TBAPF6) solution of [3]CMM•13PF6 (30 μM) was added to a BASi bulk electrolysis cell, which was equipped (Extended Data Fig. 7) with a reticular vitreous carbon working electrode, a coiled platinum-wire auxiliary electrode within a fritted glass chamber and a Ag/AgCl reference electrode, and connected to a Gamry multipurpose instrument (Reference 600) interfaced to a PC. The auxiliary electrode chamber was filled with an excess of Cu(MeCN)4PF6 dissolved in MeCN (1 ml, 0.1 M TBAPF6). The auxiliary electrode constituted a platinum wire wrapped with a copper wire (diameter 0.25 mm, 99.999% trace metals basis from Sigma-Aldrich). The experimental parameters were controlled using the software of Gamry Framework v.6.30 operating in the chronocoulometry mode. A less negative reduction potential (−0.5 V) and a less positive oxidation potential (+0.7 V) were used to limit the degradation of the [3]catenane. The whole apparatus was subjected to five redox cycles with alternate constant potentials of −0.5 V (reduction potential versus Ag/AgCl) and +0.7 V (oxidation potential versus Ag/AgCl) for 10 and 15 min, respectively.
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information files. Crystallographic data for the [3]catenane in its reduced state [3]CMM7+6• can be obtained free of charge from www.ccdc.cam.ac.uk under CCDC deposition number 2168726.
References
Astumian, R. D. Making molecules into motors. Sci. Am. 285, 56–64 (2001).
Kassem, S. et al. Artificial molecular motors. Chem. Soc. Rev. 46, 2592–2621 (2017).
Baroncini, M., Silvi, S. & Credi, A. Photo- and redox-driven artificial molecular motors. Chem. Rev. 120, 200–268 (2020).
Leigh, D. A., Wong, J. K. Y., Dehez, F. & Zerbetto, F. Unidirectional rotation in a mechanically interlocked molecular rotor. Nature 424, 174–179 (2003).
Erbas-Cakmak, S. et al. Rotary and linear molecular motors driven by pulses of a chemical fuel. Science 358, 340–343 (2017).
Odell, B. et al. Cyclobis(paraquat-p-phenylene). A tetracationic multipurpose receptor. Angew. Chem. Int. Edn Engl. 27, 1547–1550 (1988).
Astumian, R. D. & Derényi, I. Fluctuation driven transport and models of molecular motors and pumps. Eur. Biophys. J. 27, 474–489 (1998).
Chatterjee, M. N., Kay, E. R. & Leigh, D. A. Beyond switches: ratcheting a particle energetically uphill with a compartmentalized molecular machine. J. Am. Chem. Soc. 128, 4058–4073 (2006).
Mukherjee, S. & Warshel, A. The FOF1 ATP synthase: from atomistic three-dimensional structure to the rotary-chemical function. Photosynth. Res. 134, 1–15 (2017).
Astumian, R. D. & Robertson, B. Imposed oscillations of kinetic barriers can cause an enzyme to drive a chemical reaction away from equilibrium. J. Am. Chem. Soc. 115, 11063–11068 (1993).
Pezzato, C. et al. Controlling dual molecular pumps electrochemically. Angew. Chem. Int. Edn 57, 9325–9329 (2018).
Pumm, A.-K. et al. A DNA origami rotary ratchet motor. Nature 607, 492–498 (2022).
Cheng, C., McGonigal, P. R., Stoddart, J. F. & Astumian, R. D. Design and synthesis of nonequilibrium systems. ACS Nano 9, 8672–8688 (2015).
Feng, L. et al. Active mechanisorption driven by pumping cassettes. Science 374, 1215–1221 (2021).
Balzani, V., Credi, A., Raymo, F. M. & Stoddart, J. F. Artificial molecular machines. Angew. Chem. Int. Edn 39, 3348–3391 (2000).
Kay, E. R. & Leigh, D. A. Rise of the molecular machines. Angew. Chem. Int. Edn 54, 10080–10088 (2015).
Feringa, B. L. The art of building small: from molecular switches to motors (Nobel Lecture). Angew. Chem. Int. Edn 56, 11060–11078 (2017).
Sauvage, J.-P. From chemical topology to molecular machines (Nobel Lecture). Angew. Chem. Int. Edn 56, 11080–11093 (2017).
Stoddart, J. F. Mechanically interlocked molecules (MIMs)—molecular shuttles, switches, and machines (Nobel Lecture). Angew. Chem. Int. Edn 56, 11094–11125 (2017).
Abendroth, J. M., Bushuyev, O. S., Weiss, P. S. & Barrett, C. J. Controlling motion at the nanoscale: rise of the molecular machines. ACS Nano 9, 7746–7768 (2015).
Astumian, R. D. Stochastically pumped adaptation and directional motion of molecular machines. Proc. Natl. Acad. Sci. USA 115, 9405–9413 (2018).
García-López, V., Liu, D. & Tour, J. M. Light-activated organic molecular motors and their applications. Chem. Rev. 120, 79–124 (2020).
Dattler, D. et al. Design of collective motions from synthetic molecular switches, rotors, and motors. Chem. Rev. 120, 310–433 (2020).
Ragazzon, G., Baroncini, M., Silvi, S., Venturi, M. & Credi, A. Light-powered autonomous and directional molecular motion of a dissipative self-assembling system. Nat. Nanotechnol. 10, 70–75 (2015).
Cheng, C. et al. An artificial molecular pump. Nat. Nanotechnol. 10, 547–553 (2015).
Qiu, Y. et al. A precise polyrotaxane synthesizer. Science 368, 1247–1253 (2020).
Amano, S., Fielden, S. D. P. & Leigh, D. A. A catalysis-driven artificial molecular pump. Nature 594, 529–534 (2021).
Hernández, J. V., Kay, E. R. & Leigh, D. A. A reversible synthetic rotary molecular motor. Science 306, 1532–1537 (2004).
Wilson, M. R. et al. An autonomous chemically fuelled small-molecule motor. Nature 534, 235–240 (2016).
Pooler, D. R. S., Lubbe, A. S., Crespi, S. & Feringa, B. L. Designing light-driven rotary molecular motors. Chem. Sci. 12, 14964–14986 (2021).
Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
Štacko, P. et al. Locked synchronous rotor motion in a molecular motor. Science 356, 964–968 (2017).
Amano, S., Borsley, S., Leigh, D. A. & Sun, Z. Chemical engines: driving systems away from equilibrium through catalyst reaction cycles. Nat. Nanotechnol. 16, 1057–1067 (2021).
Borsley, S., Kreidt, E., Leigh, D. A. & Roberts, B. M. W. Autonomous fuelled directional rotation about a covalent single bond. Nature 604, 80–85 (2022).
Kudernac, T. et al. Electrically driven directional motion of a four-wheeled molecule on a metal surface. Nature 479, 208–211 (2011).
Tierney, H. L. et al. Experimental demonstration of a single-molecule electric motor. Nat. Nanotechnol. 6, 625–629 (2011).
Perera, U. G. E. et al. Controlled clockwise and anticlockwise rotational switching of a molecular motor. Nat. Nanotechnol. 8, 46–51 (2012).
Trabolsi, A. et al. Radically enhanced molecular recognition. Nat. Chem. 2, 42–49 (2010).
Cai, K., Zhang, L., Astumian, R. D. & Stoddart, J. F. Radical-pairing-induced molecular assembly and motion. Nat. Rev. Chem. 5, 447–465 (2021).
Pezzato, C. et al. An efficient artificial molecular pump. Tetrahedron 73, 4849–4857 (2017).
Qiu, Y. et al. A molecular dual pump. J. Am. Chem. Soc. 141, 17472–17476 (2019).
Qiu, Y., Feng, Y., Guo, Q.-H., Astumian, R. D. & Stoddart, J. F. Pumps through the ages. Chem 6, 1952–1977 (2020).
Feng, Y. et al. Molecular pumps and motors. J. Am. Chem. Soc. 143, 5569–5591 (2021).
Bruns, C. J. & Stoddart, J. F. The Nature of the Mechanical Bond: From Molecules to Machines (Wiley, 2016).
Astumian, R. D. & Bier, M. Biased Brownian motion as the operating principle for microscopic engines. Bioelectrochem. Bioenerg. 39, 67–75 (1996).
Grommet, A. B., Feller, M. & Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 15, 256–271 (2020).
Astumian, R. D. Thermodynamics and kinetics of a Brownian motor. Science 276, 917–922 (1997).
Tannor, D. J. et al. Accurate first principles calculation of molecular charge distributions and solvation energies from ab initio quantum mechanics and continuum dielectric theory. J. Am. Chem. Soc. 116, 11875–11882 (1994).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2007).
Jaguar v.10.6 (Schrödinger, Inc., 2019).
Li, H. et al. Mechanical bond-induced radical stabilization. J. Am. Chem. Soc. 135, 456–467 (2013).
Jiao, Y. et al. Electron-catalysed molecular recognition. Nature 603, 265–270 (2022).
Acknowledgements
We thank Northwestern University (NU) for its support of this research, the Integrated Molecular Structure Education and Research Center (IMSERC) at NU for providing access to equipment for relevant experiments, S. Shafaie for assistance with high-resolution mass spectrometry measurements, Y. Zhang and Y. Wu for help with NMR spectroscopic measurements and C. Cheng for useful discussions. The computational investigations at California Institute of Technology were supported by National Science Foundation grant no. CBET-2005250 (W.-G.L. and W.A.G.).
Author information
Authors and Affiliations
Contributions
J.F.S. directed the project. L.Z. and C.P. conceived the idea for the project. L.Z. designed, synthesized and characterized the compounds. W.-G.L. and W.A.G. performed density functional theory calculations. R.D.A. contributed to theoretical analyses carried out on the mechanism of operation of the electric molecular motor. Y.Q. provided some of the precursors and performed CPE experiments. H.C. contributed to the graphical design used in the figures. D.S. conducted the video recording and contributed to the animation. B.S. carried out electrospray ionization mass spectrometry and travelling-wave ion mobility mass spectrometry measurements and analyses. C.L.S. collected the single-crystal X-ray diffraction data and solved the solid-state structure. K.C., H.W., Y.J., Y.F., J.S.W.S., J.T., Y.T., X.-Y.C., Q.-H.G. and D.P. commented on the data. All the authors participated in evaluating the results. L.Z., R.D.A. and J.F.S. produced numerous drafts of the manuscript and supplementary materials, with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Junpei Yuasa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Calculated PES of the oxidized [2]catenane [2]C9+.
a, The potential energy for the CBPQT4+ ring traversing the loop in the oxidized state. The numbered atoms on the loop are used to define the position of the CBPQT4+ ring. b, Graphical representation of the oxidized state of the [2]catenane [2]C9+. c, Graphical representation of the calculated PES of the CBPQT4+ ring moving around the loop, shown in a rollercoaster manner for the fully oxidized [2]C9+.
Extended Data Fig. 2 Calculated PES of the reduced [2]catenane [2]C5+4•.
a, The potential energy for the CBPQT2(+•) ring as a function of its position on the loop in the reduced state of the [2]catenane [2]C5+4•. The numbered atoms on the loop are used to define the position of the CBPQT2(+•) ring. b, Graphical representation of the reduced state of [2]catenane [2]C5+4• in its lowest-energy co-conformation in which the CBPQT2(+•) ring encircles the V+• unit, position 10. c, Graphical representation of calculated potential energy of the CBPQT2(+•) ring as it moves around the loop, shown in a rollercoaster manner for the radical state [2]C5+4•. d, Quantum mechanical minimized structures (M06-2X/6-31G* basis set) for the CBPQT2(+•) ring located at positions 10, 21 and 27, respectively.
Extended Data Fig. 3 Calculated PES of the reduced [3]catenane [3]CMM7+6•.
a, Graphical representations of the redox process experienced by the [3]catenane in going from the oxidized state I (48, 19) to the reduced state II (28, 10). b, A two-dimensional position map describing the movement of the two reduced CBPQT2(+•) rings (A and B) around the loop. Four hypothetical paths (R1–R4) during the reduction process are illustrated by diamond (grey), circle (light red), triangle (black) and square (red) symbols, respectively. The green arrow indicates the preferred direction of movement and the red arrow indicates the less preferred (nearly precluded) direction of movement. The green, purple, black, blue and magenta lines represent the positions of the IPP, V+•, BPM, PY+ and T units, respectively. The dashed red diagonal lines represent barriers that cannot be crossed physically because doing so would require the two CBPQT2(+•) rings to occupy the same space. c, The PESs of the two CBPQT2(+•) rings moving around the loop in the reduced state, starting from point I (48, 19) and following paths R3 and R4, respectively. d, Structural formula of the loop with atoms numbered to define the positions of the CBPQT2(+•) rings. e,f, Quantum mechanical minimized structures (M06-2X/6-31G* basis set, top-down and side-on views) for the CBPQT2(+•) rings at positions (32, 10) and (2, 28), respectively.
Extended Data Fig. 4 Calculated PES of the oxidized [3]catenane [3]CMM13+.
a, Graphical representations of the process experienced by the [3]catenane in going from the oxidized state II (28, 10) to the oxidized state I′ (19, 49). b, A two-dimensional position map describing the movement of the two oxidized CBPQT4+ rings (A and B) around the loop. Four hypothetical paths O1–O4 during the oxidation process are illustrated by diamond (black), circle (red), triangle (grey) and square (light red) symbols, respectively. The green arrow indicates the preferred direction of movement and the red arrow indicates the less preferred (nearly precluded) direction of movement. The green, blue, black, blue and magenta lines represent the positions of the IPP, V2+, BPM, PY+ and T units, respectively. The dashed red diagonal line represents a barrier that cannot be crossed physically because the two CBPQT4+ rings would occupy the same space. c, Structural formula of the loop with atoms numbered to define the positions of the CBPQT4+ rings. d, The PESs of the two CBPQT4+ rings moving around the loop in the oxidized state starting from point II (28, 10) and following paths O1 and O2, respectively. The value for the energy barrier ΔE‡ of 20.1 kcal mol−1 was determined from the energy difference between the positions (19, 3) and (20, 1). The position (19, 3) corresponds to the metastable state on path O1. e,f, Quantum mechanical minimized structures (M06-2X/6-31G* basis set, top-down and side-on views) for the CBPQT4+ rings at positions (20, 1) and (34, 10), respectively. Quantum mechanical minimized structures of the lowest-energy state (19, 48) and metastable state (21, 3) are presented in Figs. 2a and 4a, respectively.
Extended Data Fig. 5 A two-dimensional position map describing the movement of two CBPQT4+/2(+•) rings around the loop.
The x and y axes represent the positions of the CBPQT4+/2(+•) rings on the loop. The green, black, blue and magenta lines represent the positions of the IPP, BPM, PY+ and T units, respectively. The hypothetical paths involving switching of the [3]catenane [3]CMM during the redox cycles are illustrated in blue (oxidation) with purple (reduction) arrows, which are periodic on account of the cyclic nature of the loop. The trajectories indicate that, for unidirectional motion, there is a Coulombic barrier (PY+) and a steric barrier (IPP) under reducing and oxidizing conditions, respectively. The dashed red diagonal line represents a barrier that cannot be crossed physically for the simple reason that two CBPQT4+/2(+•) rings would end up occupying the same space.
Extended Data Fig. 6 Scan-rate variation (0.02–2.0 V s−1) of cyclic voltammograms of [3]CMM•13PF6 (0.5 mM).
As the scan rate is increased to 2.0 V s−1, only one reduction peak for the radical state [3]CMM7+6• is observed, indicating that the electron-transfer process is much faster than ring movement at this fast scan rate. This observation suggests that, under the experimental conditions used during the operation of the [3]catenane motor, the reduction to the radical state [3]CMM7+6• and the reoxidation to the fully oxidized state [3]CMM13+ is completed fully and very rapidly.
Extended Data Fig. 7 Electrically driven operation of [3]CMM.
a, Graphical illustration of the electrochemical cell used in the repeated controlled potential electrolysis experiments. CE, counter electrode; RE, reference electrode; WE, working electrode. b, Photographs of the oxidized state (left) [3]CMM13+ and the reduced state (right) [3]CMM7+6• in the electrochemical cell.
Extended Data Fig. 8 Synthesis of the deuterium-labelled [3]catenane [Dn]-[3]CMM.
By sequentially adding [D16]-CBPQT2(+•) and CBPQT2(+•) during the synthesis of [Dn]-[3]CMM, it is able to bias the distribution of the undeuterated CBPQT4+ rings on the T and BPM units. The [3]catenane [Dn]-[3]CMM13+ was obtained as a mixture of four isotopologues, including [D0]-[3]CMM13+, and [D32]-[3]CMM13+, in addition to two co-constitutional isomers [D16]-[3]CMM13+. CuAAC, copper(I)-catalysed azide–alkyne cycloaddition.
Extended Data Fig. 9 Calculation of the probability of 180° rotation for both rings on the loop.
Distribution of CBPQT4+ rings on the two positions (T and BPM) of the cyclic track according to the 1H NMR spectra (Fig. 3) of [Dn]-[3]CMM•13PF6 before and after one redox cycle, based on the relevant integration of protons H-Phen″ (I1/I1′) and H-Phen′ (I2/I2′), respectively. η, the probability of 180° rotation for both rings on the loop.
Extended Data Fig. 10 Probable direction of the movement of two CBPQT4+/2(+•) rings during the redox operation of [3]CMM13+.
Clockwise means that each CBPQT4+/2(+•) ring sees the substituents on the loop in the order T → PY+ → V2+/+• → V2+/+• → IPP → T and counterclockwise means that each ring sees the substituents on the loop in the order T → IPP → V2+/+• → V2+/+• → PY+ → T. The curly arrows represent the direction in which the two rings move around the loop. The ‘pred’ and ‘pox’ near the arrows are the probability of clockwise motion of the two rings on the loop in the redox cycle. The kred,+, kred,−, kox,− and kox,+ are the corresponding rate constants for the probable steps during the redox cycle, respectively, and the clockwise and counterclockwise steps are also indicated by ‘+’ and ‘−’, respectively. The 180° rotation is represented by ‘1/2’.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–78, Schemes 1–9, Tables 1–11, supplementary text and notes, extensive experimental data, quantum mechanical calculation results and detailed discussions.
Supplementary Video 1
Video of controlled potential electrolysis of the [3]catenane molecular motor in an electrochemical cell.
Supplementary Video 2
Animation of the unidirectional rotary motion in the [3]catenane molecular motor during a redox cycle.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, L., Qiu, Y., Liu, WG. et al. An electric molecular motor. Nature 613, 280–286 (2023). https://doi.org/10.1038/s41586-022-05421-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05421-6
This article is cited by
-
Analytical noncovalent electrochemistry for battery engineering
Nature Chemical Engineering (2024)
-
Artificial molecular pumps
Nature Reviews Methods Primers (2024)
-
Animating hydrogel knotbots with topology-invoked self-regulation
Nature Communications (2024)
-
Ratcheting synthesis
Nature Reviews Chemistry (2023)
-
Thermally-induced atropisomerism promotes metal-organic cage construction
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.