Abstract
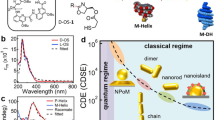
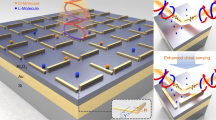
Quantitative determination and in situ monitoring of molecular chirality at extremely low concentrations is still challenging with simple optics because of the molecular-scale mismatch with the incident light wavelength. Advances in spectroscopy1,2,3,4 and nanophotonics have successfully lowered the detection limit in enantioselective sensing, as it can bring the microscopic chiral characteristics of molecules into the macroscopic scale5,6,7 or squeeze the chiral light into the subwavelength scale8,9,10,11,12,13,14,15,16,17. Conventional nanophotonic approaches depend mainly on the optical helicity density8,9 by localized resonances within an individual structure, such as localized surface plasmon resonances (LSPRs)10,11,12,13,14,15,16 or dielectric Mie resonances17. These approaches use the local chiral hotspots in the immediate vicinity of the structure, whereas the handedness of these hotspots varies spatially. As such, these localized resonance modes tend to be error-prone to the stochasticity of the target molecular orientations, vibrations and local concentrations18,19. Here we identified enantioselective characteristics of collective resonances (CRs)20 arising from assembled 2D crystals of isotropic, 432-symmetric chiral gold nanoparticles (helicoids)21,22. The CRs exhibit a strong and uniform chiral near field over a large volume above the 2D crystal plane, resulting from the collectively spinning, optically induced dipoles at each helicoid. Thus, energy redistribution by molecular back action on the chiral near field shifts the CRs in opposite directions, depending on the handedness of the analyte, maximizing the modulation of the collective circular dichroism (CD).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data that support the findings (including source data for main figures) of this study are available from the corresponding authors on request.
Code availability
All codes generated during this study are available from the corresponding authors on request.
References
Fasman, G. D. Circular Dichroism and the Conformational Analysis of Biomolecules (Springer, 1996).
Barron, L. D. Molecular Light Scattering and Optical Activity (Cambridge Univ. Press, 2004).
Ranjbar, B. & Gill, P. Circular dichroism techniques: biomolecular and nanostructural analyses- a review. Chem. Biol. Drug Des. 74, 101–120 (2009).
Rhee, H. et al. Femtosecond characterization of vibrational optical activity of chiral molecules. Nature 458, 310–313 (2009).
Ma, W. et al. Attomolar DNA detection with chiral nanorod assemblies. Nat. Commun. 4, 2689–2696 (2013).
Han, B., Zhu, Z., Li, Z., Zhang, W. & Tang, Z. Conformation modulated optical activity enhancement in chiral cysteine and Au nanorod assemblies. J. Am. Chem. Soc. 136, 16104–16107 (2014).
Kuzyk, A. et al. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 483, 311–314 (2012).
Lipkin, D. M. Existence of a new conservation law in electromagnetic theory. J. Math. Phys. 5, 696–700 (1964).
Tang, Y. & Cohen, A. E. Optical chirality and its interaction with matter. Phys. Rev. Lett. 104, 163901–163904 (2010).
Govorov, A. O., Fan, Z., Hernandez, P., Slocik, J. M. & Naik, R. R. Theory of circular dichroism of nanomaterials comprising chiral molecules and nanocrystals: plasmon enhancement, dipole interactions, and dielectric effects. Nano Lett. 10, 1374–1382 (2010).
Schäferling, M., Yin, X. & Giessen, H. Formation of chiral fields in a symmetric environment. Opt. Express 20, 26326–26336 (2012).
Davis, T. J. & Hendry, E. Superchiral electromagnetic fields created by surface plasmons in nonchiral metallic nanostructures. Phys. Rev. B 87, 085405 (2013).
Yoo, S. & Park, Q. H. Chiral light-matter interaction in optical resonators. Phys. Rev. Lett. 114, 203003 (2015).
Abdulrahman, N. A. et al. Induced chirality through electromagnetic coupling between chiral molecular layers and plasmonic nanostructures. Nano Lett. 12, 977–983 (2012).
Hendry, E. et al. Ultrasensitive detection and characterization of biomolecules using superchiral fields. Nat. Nanotechnol. 5, 783–787 (2010).
Probst, P. T. et al. Mechano-tunable chiral metasurfaces via colloidal assembly. Nat. Mater. 20, 1024–1028 (2021).
Solomon, M. L., Hu, J., Lawrence, M., García-Etxarri, A. & Dionne, J. A. Enantiospecific optical enhancement of chiral sensing and separation with dielectric metasurfaces. ACS Photonics 6, 43–49 (2019).
Choi, J. S. & Cho, M. Limitations of a superchiral field. Phys. Rev. A 86, 063834 (2012).
Hentschel, M., Schäferling, M., Duan, X., Giessen, H. & Liu, N. Chiral plasmonics. Sci. Adv. 3, e1602735 (2017).
Auguié, B. & Barnes, W. L. Collective resonances in gold nanoparticle arrays. Phys. Rev. Lett. 101, 143902 (2008).
Lee, H.-E. et al. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 556, 360–365 (2018).
Im, S. W. et al. Chiral surface and geometry of metal nanocrystals. Adv. Mater. 32, 1905758–1905777 (2020).
Schäferling, M., Dregely, D., Hentschel, M. & Giessen, H. Tailoring enhanced optical chirality: design principles for chiral plasmonic nanostructures. Phys. Rev. X 2, 031010–031018 (2012).
Ahn, H. Y. et al. Bioinspired toolkit based on intermolecular encoder toward evolutionary 4D chiral plasmonic materials. Acc. Chem. Res. 52, 2768–2783 (2019).
Arteaga, O. et al. Relation between 2D/3D chirality and the appearance of chiroptical effects in real nanostructures. Opt. Express 24, 2242–2252 (2016).
Eismann, J. S., Neugebauer, M. & Banzer, P. Exciting a chiral dipole moment in an achiral nanostructure. Optica 5, 954–959 (2018).
Kittel, C. & McEuen, P. Introduction to Solid State Physics (Wiley, 1996).
Chen, H., Kou, X., Yang, Z., Ni, W. & Wang, J. Shape- and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 24, 5233–5237 (2008).
Jung, L. S., Campbell, C. T., Chinowsky, T. M., Mar, M. N. & Yee, S. S. Quantitative interpretation of the response of surface plasmon resonance sensors to adsorbed films. Langmuir 14, 5636–5648 (1998).
Ryu, J.-K. et al. Spring-loaded unraveling of a single SNARE complex by NSF in one round of ATP turnover. Science 347, 1485–1489 (2015).
Kim, C. et al. Extreme parsimony in ATP consumption by 20S complexes in the global disassembly of single SNARE complexes. Nat. Commun. 12, 3206 (2021).
Šípová, H. et al. Surface plasmon resonance biosensor for rapid label-free detection of microribonucleic acid at subfemtomole level. Anal. Chem. 82, 10110–10115 (2010).
Sreekanth, K. V. et al. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 15, 621–627 (2016).
Park, J. H. et al. Symmetry-breaking-induced plasmonic exceptional points and nanoscale sensing. Nat. Phys. 16, 462–468 (2020).
Liang, B., Kiessling, V. & Tamm, L. K. Prefusion structure of syntaxin-1A suggests pathway for folding into neuronal trans-SNARE complex fusion intermediate. Proc. Natl Acad. Sci. USA 110, 19384–19389 (2013).
Nusz, G. J. et al. Label-free plasmonic detection of biomolecular binding by a single gold nanorod. Anal. Chem. 80, 984–989 (2008).
Nelson, S. G., Johnston, K. S. & Yee, S. S. High sensitivity surface plasmon resonance sensor based on phase detection. Sens. Actuators B Chem. 35, 187–191 (1996).
Nelson, B. P., Grimsrud, T. E., Liles, M. R., Goodman, R. M. & Corn, R. M. Surface plasmon resonance imaging measurements of DNA and RNA hybridization adsorption onto DNA microarrays. Anal. Chem. 73, 1–7 (2001).
Zhao, Y. et al. Chirality detection of enantiomers using twisted optical metamaterials. Nat. Commun. 8, 14180 (2017).
Wu, H. L. et al. A comparative study of gold nanocubes, octahedra, and rhombic dodecahedra as highly sensitive SERS substrates. Inorg. Chem. 50, 8106–8111 (2011).
Odom, T. W., Love, J. C., Wolfe, D. B., Paul, K. E. & Whitesides, G. M. Improved pattern transfer in soft lithography using composite stamps. Langmuir 18, 5314–5320 (2002).
Lee, H. E. et al. Cysteine-encoded chirality evolution in plasmonic rhombic dodecahedral gold nanoparticles. Nat. Commun. 11, 263 (2020).
Zhang, X., Servos, M. R. & Liu, J. Instantaneous and quantitative functionalization of gold nanoparticles with thiolated DNA using a pH-assisted and surfactant-free route. J. Am. Chem. Soc. 134, 7266–7269 (2012).
Rhee, H., Choi, J. S., Starling, D. J., Howell, J. C. & Cho, M. Amplifications in chiroptical spectroscopy, optical enantioselectivity, and weak value measurement. Chem. Sci. 4, 4107–4114 (2013).
Shafiei, F. et al. A subwavelength plasmonic metamolecule exhibiting magnetic-based optical Fano resonance. Nat. Nanotechnol. 8, 95–99 (2013).
Gurvitz, E. A. et al. The high-order toroidal moments and anapole states in all-dielectric photonics. Laser Photonics Rev. 13, 1800266 (2019).
Lindell, I. V., Vitanen, A. J. & Tretyakov, S. A. Electromagnetic Waves in Chiral and Bi-Isotropic Media (Artech House Publishers, 1994).
Shahmoon, E., Wild, D. S., Lukin, M. D. & Yelin, S. F. Cooperative resonances in light scattering from two-dimensional atomic arrays. Phys. Rev. Lett. 118, 113601–113606 (2017).
Zhang, W. & Martin, O. J. F. A universal law for plasmon resonance shift in biosensing. ACS Photonics 2, 144–150 (2015).
Wang, F. & Shen, Y. R. General properties of local plasmons in metal nanostructures. Phys. Rev. Lett. 97, 206806 (2006).
Acknowledgements
This research was supported by the Creative Materials Discovery Program (2017M3D1A1039377) through the National Research Foundation of Korea (NRF) financed by the Ministry of Science and ICT (MSIT). K.T.N. thanks the Technology Innovation Program (20012390) of the Ministry of Trade, Industry and Energy (MOTIE), LG Display, Institute of Engineering Research, Research Institute of Advanced Materials (RIAM), SOFT Foundry Institute and NRF-MSIT grant 2021R1A4A5031762. S.L., Q.-H.P. and S.Y. are grateful for the NRF-MSIT grant (2019R1A4A1028121). S.L. appreciates the support from the KU-KIST School Project, Korea University grant and NRF (2019R1A2C2004846). S.L. and Q.-H.P. thank the Basic Research Laboratory program (2022R1A4A1034315). S.Y. is thankful for the NRF-MSIT grant (2022R1F1A1074019). T.-Y.Y. thanks the Bio & Medical Technology Development Program (2018M3A9E2023523). R.M.K. and H.K. are grateful for the Global Ph.D. Fellowship Program (2019H1A2A1077150 and 2019H1A2A1077048).
Author information
Authors and Affiliations
Contributions
K.T.N. and R.M.K. conceived the idea and obtained the initial results. R.M.K., J.-H.H., S.Y., T.G.K., C.K., T.-Y.Y., Q.-H.P., S.L. and K.T.N. designed the research and wrote the manuscript. R.M.K., H.K., J.H.H. and N.H.C. synthesized, characterized and fabricated the materials. J.R.J. and M.H.L. discussed and helped with the preparation of the master mould for the PDMS pattern. Q.-H.P. and S.Y. theorized the results. J.-H.H., S.Y., E.I. and S.L. performed numerical simulation and analysed the data. R.M.K., J.-H.H., Y.-C.L. and S.W.I. conducted optical characterization of materials. T.G.K., C.K. and T.-Y.Y. prepared materials for SNARE complexes and contributed to the reconstitution essay of SNARE complexes. H.-Y.L. supported the preparation and characterization of nucleotides. All authors discussed the experiments and contributed to writing the manuscript. Q.-H.P., S.Y. and S.L. guided numerical simulation and theoretical demonstration of the work. K.T.N. guided all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Bart Kahr and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Simulation of the optical properties of 2D helicoid crystals.
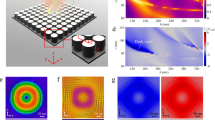
a, Photonic band structures of the 2D helicoid crystal. The CRs and LSPRs are highlighted by thick, colour-filled circles, whereas other modes are plotted by unfilled circles. Blue and red circles correspond to the TE and TM modes, respectively. The CRs vary steeply for different in-plane momenta, whereas the LSPRs do not because they are localized resonances insensitive to the in-plane momenta. The TE and TM modes of 2D helicoid crystal overlaps at the 60° slanted incidence of light along the Γ to M symmetry point. b, Phase difference of the dipole moment of helicoids in the crystal along the TE (pTE) and TM (pTM) directions (that is, arg(pTE/pTM)/π in the crystal plane). In the condition of the CR (blue region), the phase difference of pTE and pTM is ±π/2 (dashed red and blue lines), whereas other conditions generate either phase difference of pTE and pTM 0 or −π. As a result, the helicoid dipoles (p) of crystal collectively spin. c, Profiles of optical helicity density hsca(r) at the LSPRs (670 nm) of the 2D helicoid crystal. The sign of hsca(r) is alternately changing from blue (−) to red (+) near the helicoid at the LSPRs (670 nm). Thus, different from the CR mode (965 nm) of 2D helicoid crystal, no uniform hsca(r) is generated. d, Photonic band structure of the 2D gammadion crystal. e, Phase difference of the dipole moment of the gammadion in the crystal along the TE (pTE) and TM (pTM) directions (that is, arg(pTE/pTM)/π in the crystal plane). f, Profiles of the optical helicity density hsca(r) at the CR (935 nm) of the 2D gammadion crystal. The sign of hsca(r) is alternately changing from blue (−) to red (+) near the gammadion at the CR (935 nm).
Extended Data Fig. 2 Energy band diagram of the CR mode for a 2D helicoid crystal depending on LCP and RCP light in two different enantiomeric media.
The discrete energy state of the CR mode with E0+ and E0− is formed for LCP (+) and RCP (−) excitation as a result of the chiroptical properties of the 2D helicoid crystal in a chiral molecular medium with a chirality parameter of κ = 0 (racemic mixture) (dashed black lines). In the case of positive Δκ, the more negative shift of the energy state of the CR mode for RCP illumination (ΔE−(+Δκ)) than that of the CR mode for LCP illumination (ΔE+(+Δκ)) results in positive energy dichroism (ED ≡ E+(+Δκ) − E−(+Δκ) = (E(0+) − E(0−)) + (ΔE−(+Δκ) − ΔE+(+Δκ))). By contrast, D-molecules (κ < 0) lead to the opposite energy dichroism.
Extended Data Fig. 3 Fabrication and characterization of 2D helicoid crystals with helicoids.
a, Schematic illustration showing the fabrication process of 2D helicoid crystals. Water–organic phase assembly of a helicoid monolayer (i), transfer of the monolayer using dip-coating (ii) and insertion of nanoparticles by rubbing with a Teflon-coated stick (iii) was sequentially conducted. b,c, SEM images of nanopatterned PDMS (without helicoids) with 400-nm pitch, 100-nm-sized wells (b) and a 2D helicoid crystal with a large area (c). Inset of b, DFOM image of nanopatterned PDMS. d, Photograph of a fabricated 2D helicoid crystal (area: 6 cm2), e, DFOM image of the 2D helicoid crystal with a large area. Inset of e, FFT image.
Extended Data Fig. 4 Validation of our home-built ORD and CD setup by comparison with a commercial ORD and CD setup.
a, ORD spectra of 1.0 M L-proline and D-proline solutions measured in the home-built ORD measurement setup (i) and by a commercial ORD spectrophotometer (ii). b, CD spectrum of a colloidal helicoid solution. The ORD spectra (purple line), which were collected in the home-built setup, are transformed to CD using the K-K relation (black line). The red line corresponds to the CD spectra measured by the commercially available CD spectrophotometer. The K-K transformed and measured CD spectra are exactly same.
Extended Data Fig. 5 Formation and optimization of collective CD in 2D helicoid crystals for high enantioselective sensing performance.
a, SEM images of helicoids with different morphologies. The size of each helicoid is indicated in the images. A decreasing seed concentration results in an increased size of helicoids. Scale bars, 200 nm. b, CD spectra of the colloidal solutions of synthesized helicoids (i) and corresponding CD spectra of 2D helicoid crystals (ii). Gradually redshifted CD peak positions with similar CD intensities are observed for size-increasing helicoids in colloidal solutions. The same redshifting trend for CD peaks is observed in 2D helicoid crystals with increasing helicoid size. The CD intensities of 2D helicoid crystals are not proportional to those of colloidal solutions because the excitation efficiency of the CR and the resulting collective CD can be tuned and optimized by controlling the size of plasmonic units in 2D crystal lattices. The intensity of the collective CD exhibits a maximum value for 180-nm-sized helicoids. c, CD intensities of 2D helicoid crystals at the maximum absolute value (|CD|max). The 2D helicoid crystal made with 180-nm-sized helicoids exhibits a ten times stronger |CD|max value than the 200-nm-sized helicoids. d, Schematic diagram of an arbitrary hexagonal plasmonic crystal and the set of incidence angles (θ, φ). Gold spheres with and without transparency indicate the position of the arbitrary plasmonic units in the plasmonic crystal before and after rotation, respectively. e, Schematic illustration of the angle-resolved excitation and a 3D-printed sample mounted at θ = 0° (left) and θ > 0° (right). Transmittance (T+, −) (f) and corresponding CD spectra (g) for different sets of incidence angles: (θ, φ) = (60°, 0°), (60°, 30°) and (60°, 90°) correspond to Γ–M, Γ–K and M–K symmetry, respectively. The spectral regions of the CR are highlighted by blue boxes. h, δλ0(κ), the mode shift, for each mode. The theoretical predictions with numerical results show that (θ, φ) = (60°, 0°) with Γ–M symmetry provides the largest mode shift induced by the molecular chirality κ.
Extended Data Fig. 6 Collective CD response for sensing molecular chirality and determining the relative ratio and concentration of enantiomeric solutions in a cuvette system.
a, Schematic illustration of sensing experiments in a cuvette system. The 2D helicoid crystal (θ, ϕ) = (60°, 0°) was loaded on the holder and inserted in a cuvette (light path of about 8.9 mm) containing a molecular solution. b, Full-range CD spectra of 2D helicoid crystals immersed in DW and 0.1 M–1.0 M L-Pro and D-Pro. The sensitivity of the LSPR (approximately 650 nm) is lower than that of the collective CD (approximately 825 and 910 nm), as the peak positions of the LSPR remain constant with the introduction of L-Pro and D-Pro. c, Change in collective CD induced by 1.0 M L-Pro and D-Pro (ΔCD ≡ CDMolecule − CDDW). The overall changes in the collective CD with respect to both the spectral position and intensity are quantified by calculating the difference in ΔCD at the peak and dip positions (ΔCDmax − ΔCDmin). The introduction of 1.0 M D-Pro substantially modulates the collective CD and collective mode of the 2D helicoid crystal than the introduction of 1.0 M L-Pro. d, Plane equations for Region I and Region II of the collective CD shown in Fig. 3c. Region I and Region II in (i) can be expressed as plane I: ax + by + cz = d and plane II: a′x + b′y + c′z = d′ (ii). The x, y and z axes correspond to the relative ratio of D-molecules (for example, 8:2 corresponds to x = 0.2), total concentration of enantiomeric solution and shift value, respectively. The constants a (a′), b (b′) and c (c′) are intrinsically determined by the molecules. Three representative points constituting each plane were introduced into the equations of plane I and plane II (ii). Solving simultaneous equations, the constants for proline molecules can be obtained as a (a′) = −16.2 (7.8), b (b′) = 15.9 (8.5), c (c′) = −1 (−1) and d (d′) = 0 (−7.8). The compositions of enantiomeric solutions at the boundary of plane I and plane II (grey circles) can be precisely determined by using the obtained equations. e, CD spectra of 2D helicoid crystals immersed in DW and 1.0 M proline solution. The proline solution was composed of L-proline and D-proline at an 8:2 ratio. The L:D ratio in solution and total concentration (inset) calculated by inserting the shift values of each region into the obtained plane equations closely coincide with the actual composition and concentration of the solution. f, CD spectra of 2D helicoid crystals immersed in DW (black) and 0.5 M or 1.0 M L-glucose (red, L-Glu) and D-glucose (blue, D-Glu) in the collective CD range. The red (blue) circle represents the spectral position of the collective CD in Region I (Region II). The same trend of region-sensitive collective CD shift (arrows) is observed for glucose molecules (Region I (red): L-Glu-sensitive; Region II (blue): D-Glu-sensitive).
Extended Data Fig. 7 Enantioselective detection of chiral biomolecules in an analyte chamber system of small volume.
a, Schematic illustration presenting the fabrication procedure of an analyte chamber with lowered analyte height (150 μm) and volume (15 μl). Two types of triangular prism structure, the bottom (i, left) layer without an inlet or an outlet and the top (i, right) layer with an inlet and an outlet, were fabricated by moulding a 3D-printed mould with PDMS (ii). The fabricated structure was detached from the mould, exhibiting a 60° slanted angle for incident light (θ = 60°) (iii). A 2D helicoid crystal of Γ–M (φ = 0°) symmetry was loaded on the bottom layer, and a spacer with a thickness of 150 µm was attached on top of the 2D helicoid crystal (iv). Finally, all of the structures were assembled by masking the empty space of the assembled structure with PDMS (v). For the detailed procedure, see Methods. b, CD spectra of 2D helicoid crystals in the analyte chamber containing different solutions. DW, 0.1 M L-Pro and 0.1 M D-Pro solutions are indicated by black, red and blue lines, respectively. Inset, magnified CD spectrum. The CD intensity decreases with molecular introduction, exhibiting a larger change for D-proline. c, ΔCD ≡ CDMolecule − CDDW spectra of 2D helicoid crystals for the introduction of 0.1 and 1.0 M L-Pro and D-Pro into the analyte chamber. d, Absolute value of the ΔCD minimum (|ΔCDmin|) for various concentrations of L-Pro (red triangle) and D-Pro (blue triangle) (10−6–10−1 M). Error bars included in d are plotted on the basis of statistical analysis of three multiple acquisitions. e, ΔCD ≡ CDMolecule − CDDW spectra before and after FFT filtering. The repeated noise of ΔCD was greatly reduced, sustaining the spectral features of the original spectrum. f, Numerically simulated ΔCD spectra in media with modelled concentrations of (n, κ) = (1.352, ±1.2 × 10−3) and (1.373, ±2.4 × 10−3). g, Corresponding |ΔCDmin| with modelled concentration (n, κ) = (1; 1.331, ±1.2 × 10−7), (2; 1.332, ±1.2 × 10−6), (3; 1.333, ±1.2 × 10−5), (4; 1.339, ±1.2 × 10−4), (5; 1.352, ±1.2 × 10−3) and (6; 1.373, ±2.4 × 10−3).
Extended Data Fig. 8 Collective CD for the detection of chiral nucleotides.
a, Schematic showing the quantification of ssDNA reacted with helicoids. The initial amount of thiolated ssDNA was 7.5 × 10−11 mol (Abs: 0.134). The 0.002 decrease in Abs corresponds to 1.12 × 10−12 mol (6.7 × 1011 strands), which is the total amount of thiolated ssDNA that reacted with 2D helicoid crystals. The number of helicoids in the 1-cm2 2D helicoid crystal is 4.4 × 108. This results in roughly 1,000 thiolated ssDNA per nanoparticle. b, Ultraviolet–visible response for the solution of thiolated (-SH) ssDNA before and after the reaction. The path length is 1 mm. λ260 nm is marked with a dashed black line. c, CD spectra of thiolated ssDNA-functionalized and miR-21-hybridized 2D helicoid crystals. The hybridization of miR-21 (2.5 nM) to thiolated ssDNA decreases the collective CD intensity. Inset, magnified CD spectrum. d, ΔCD ≡ CDDNA+RNA − CDDNA spectra of 2D helicoid crystals for hybridization of miR-21 with thiolated ssDNA on the 2D helicoid crystal. A gradual increase in the negativity of the ΔCD spectra at approximately 875 nm is clearly observed for a representative concentration range of 0–2.5 nM.
Extended Data Fig. 9 In vitro reconstitution assay of the SNARE complex on a 2D helicoid crystal.
a, SNARE complexes (syntaxin-1A without the N-terminal Habc domain (syntaxin, 183–288), full-length SNAP-25 isoform A (SNAP25, 1–206), soluble synaptobrevin-2/VAMP2 (sVAMP2, 1–96) and C-terminal VAMP2 (ΔN VAMP2, 49–96)) forming SNARE or acceptor complexes used in the assay. b, Schematic illustration presenting the transition of the SNARE complex from the trans to cis form during Ves–membrane fusion. c, Schematic of the in vitro reconstitution assay of SNARE complex assembly between helicoids. NTV (cyan), lipid Ves (grey) with the acceptor complex and soluble VAMP (sVAMP2, blue fragment) are sequentially added and bound to a BSA-biotin functionalized between helicoids, inducing a helix structure in complex. The SNARE structure changes from trans to cis form. d, NTV binding on BSA-biotinylated 2D helicoid crystals. Experimental scheme for observing the binding of Cy3-labelled avidin (streptavidin) to BSA-biotin on 2D helicoid crystals (i). Cy3 intensity of streptavidin on a 2D helicoid crystal (ii). Streptavidin binds to BSA-biotin but does not bind to BSA, suggesting that the biotin–NTV system can work on the 2D helicoid crystals. e, Ves delivery on NTV of a 2D helicoid crystal. Experimental scheme for observing the binding of DII-labelled Ves to NTV on BSA-biotin-bound 2D helicoid crystals (i). DII intensity of the Ves on a 2D helicoid crystal (ii). Ves bind to the 2D helicoid crystal only when NTV is added, suggesting that Ves are successfully mounted on the 2D helicoid crystal in an NTV-dependent manner. All the error bars included in d and e are plotted on the basis of statistical analysis of five multiple acquisitions. f, SNARE complex assembly on a 2D helicoid crystal. Experimental scheme for observing SNARE complex assembly on 2D helicoid crystals following the addition of Cy3-labelled sVAMP2 to the Ves-incorporated acceptor complex (i). Cy3 images of sVAMP2 on a 2D helicoid crystal (ii). Cy3-labelled sVAMP2 (200 nM) was sequentially added following the addition of 5 nM (top left) or 10 nM (top right) Ves without the acceptor complex or 5 nM (bottom left) or 10 nM (bottom right) Ves with the acceptor complex. sVAMP2 only binds to Ves incorporating the acceptor complex. In addition, the Cy3 intensity of sVAMP2 increases as more Ves are added, suggesting that the SNARE complex is successfully assembled on the 2D helicoid crystal. g,h, ΔCD ≡ CDafter conjugation − CDinitial spectra of 2D helicoid crystals for sequential conjugation of constituent proteins (NTV, Ves and sVAMP2). The CDinitial corresponds to the CD response of BSA-biotin-functionalized 2D helicoid crystal. Two types of 2D helicoid crystal with (g) and without (h) acceptor complexes in Ves were used. The addition of sVAMP2 to the 2D helicoid crystal with acceptor complexes changes the ΔCD response of the 2D helicoid crystal. The ΔCD response remains constant for the 2D helicoid crystal without the acceptor complex in Ves.
Extended Data Fig. 10 Enantioselective sensing with a 2D helicoid crystal in reflection mode with a CPL-based SPR sensing setup.
a, Photograph of the light source used in the CPL-based SPR system. The light source was focused onto a 2D helicoid crystal of size 200 μm. b, Reflectivity spectra of drop-coated helicoids on quartz substrates and 2D helicoid crystals illuminated with LCP and RCP light. The clear feature of the CR mode was observed in the reflectivity spectra of 2D helicoid crystals (dashed black box). c, Reflectivity-based CD (CDR) spectra for 2D helicoid crystals on which DW, 1.0 M L-Pro and 1.0 M D-Pro were loaded. The introduction of chiral molecules onto the 2D helicoid crystal shifts the CDR. In agreement with the transmission-based collective CD response, the 2D helicoid crystal more sensitively responds to 1.0 M D-Pro. d, Comparison of the enantioselective sensing performance of collective CD-based sensing in terms of measurable concentration range and size (that is, molecular weight) with previously reported enantioselective sensors14,15,16,50. The broad measurable range and versatile analyte selection of the 2D helicoid-crystal-based enantioselective sensor is shown by red-coloured symbols.
Supplementary information
Supplementary Video 1
Numerically retrieved temporal evolution of collective spinning dipoles (p) and scattered electric field (Esca) vectors on 2D helicoid crystal. Perspective and top view of collectively rotating helicoid dipoles p (red arrows on each helicoid) and scattered electric field (Esca) vectors (black arrows) at CR mode under 60° slanted incidence of LCP light (ELCP) to the direction of k vector (blue arrow).
Supplementary Video 2
Optical helicity distributions at CR mode in 2D helicoid crystal. The spatial distributions of optical helicity (hsca(r)) under the incidence of LCP light (ELCP) to the direction of k vector (blue arrow) at CR mode. The hsca(r) along the xy plane for different z positions is obtained and swept. The red/blue colours of hsca(r) indicate the left (+)/right (−) handedness, respectively.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, R.M., Huh, JH., Yoo, S. et al. Enantioselective sensing by collective circular dichroism. Nature 612, 470–476 (2022). https://doi.org/10.1038/s41586-022-05353-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05353-1
This article is cited by
-
Assembly of short amphiphilic peptoids into nanohelices with controllable supramolecular chirality
Nature Communications (2024)
-
Progress and prospects of chiral nanomaterials for biosensing platforms
Rare Metals (2024)
-
Coherent multipolar amplification of chiroptical scattering and absorption from a magnetoelectric nanoparticle
Communications Physics (2023)
-
Halide-assisted differential growth of chiral nanoparticles with threefold rotational symmetry
Nature Communications (2023)
-
Chiral AuCu heterostructures with site-specific geometric control and tailored plasmonic chirality
Science China Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.