Abstract
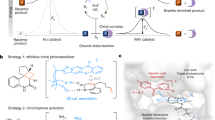
The ability to program new modes of catalysis into proteins would allow the development of enzyme families with functions beyond those found in nature. To this end, genetic code expansion methodology holds particular promise, as it allows the site-selective introduction of new functional elements into proteins as noncanonical amino acid side chains1,2,3,4. Here we exploit an expanded genetic code to develop a photoenzyme that operates by means of triplet energy transfer (EnT) catalysis, a versatile mode of reactivity in organic synthesis that is not accessible to biocatalysis at present5,6,7,8,9,10,11,12. Installation of a genetically encoded photosensitizer into the beta-propeller scaffold of DA_20_00 (ref. 13) converts a de novo Diels–Alderase into a photoenzyme for [2+2] cycloadditions (EnT1.0). Subsequent development and implementation of a platform for photoenzyme evolution afforded an efficient and enantioselective enzyme (EnT1.3, up to 99% enantiomeric excess (e.e.)) that can promote intramolecular and bimolecular cycloadditions, including transformations that have proved challenging to achieve selectively with small-molecule catalysts. EnT1.3 performs >300 turnovers and, in contrast to small-molecule photocatalysts, can operate effectively under aerobic conditions and at ambient temperatures. An X-ray crystal structure of an EnT1.3-product complex shows how multiple functional components work in synergy to promote efficient and selective photocatalysis. This study opens up a wealth of new excited-state chemistry in protein active sites and establishes the framework for developing a new generation of enantioselective photocatalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Coordinates and structure factors have been deposited in the Protein Data Bank under accession numbers 7ZP5, 7ZP6 and 7ZP7. The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Source data are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Burke, A. J. et al. Design and evolution of an enzyme with a non-canonical organocatalytic mechanism. Nature 570, 219–223 (2019).
Drienovská, I., Mayer, C., Dulson, C. & Roelfes, G. A designer enzyme for hydrazone and oxime formation featuring an unnatural catalytic aniline residue. Nat. Chem. 10, 946–952 (2018).
Chin, J. W. Expanding and reprogramming the genetic code. Nature 550, 53–60 (2017).
Liu, C. C. & Schultz, P. G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 79, 413–444 (2010).
Großkopf, J., Kratz, T., Rigotti, T. & Bach, T. Enantioselective photochemical reactions enabled by triplet energy transfer. Chem. Rev. 122, 1626–1653 (2022).
Strieth-Kalthoff, F. & Glorius, F. Triplet energy transfer photocatalysis: unlocking the next level. Chem 6, 1888–1903 (2020).
Tröster, A., Bauer, A., Jandl, C. & Bach, T. Enantioselective visible-light-mediated formation of 3-cyclopropylquinolones by triplet-sensitized deracemization. Angew. Chem. Int. Ed. 58, 3538–3541 (2019).
Kleinmans, R. et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022).
Alonso, R. & Bach, T. A chiral thioxanthone as an organocatalyst for enantioselective [2+2] photocycloaddition reactions induced by visible light. Angew. Chem. Int. Ed. 53, 4368–4371 (2014).
Münster, N., Parker, N. A., van Dijk, L., Paton, R. S. & Smith, M. D. Visible light photocatalysis of 6π heterocyclization. Angew. Chem. Int. Ed. 56, 9468–9472 (2017).
Huang, M., Zhang, L., Pan, T. & Luo, S. Deracemization through photochemical E/Z isomerization of enamines. Science 375, 869–874 (2022).
Maturi, M. M., Pöthig, A. & Bach, T. Enantioselective photochemical rearrangements of spirooxindole epoxides catalyzed by a chiral bifunctional xanthone. Aust. J. Chem. 68, 1682–1692 (2015).
Siegel, J. B. et al. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction. Science 329, 309–313 (2010).
Becker, M. R., Richardson, A. D. & Schindler, C. S. Functionalized azetidines via visible light-enabled aza Paternò-Büchi reactions. Nat. Commun. 10, 5095 (2019).
Müller, C. et al. Enantioselective intramolecular [2 + 2]-photocycloaddition reactions of 4-substituted quinolones catalyzed by a chiral sensitizer with a hydrogen-bonding motif. J. Am. Chem. Soc. 133, 16689–16697 (2011).
Vogt, F., Jödicke, K., Schröder, J. & Bach, T. Paternò-Büchi reactions of silyl enol ethers and enamides. Synthesis 24, 4268–4273 (2009).
Blum, T. R., Miller, Z. D., Bates, D. M., Guzei, I. A. & Yoon, T. P. Enantioselective photochemistry through Lewis acid-catalyzed triplet energy transfer. Science 354, 1391–1395 (2016).
Mayr, F., Brimioulle, R. & Bach, T. A chiral thiourea as a template for enantioselective intramolecular [2 + 2] photocycloaddition reactions. J. Org. Chem. 81, 6965–6971 (2016).
Hörman, F. M. et al. Triplet energy transfer from ruthenium complexes to chiral eniminium ions: enantioselective synthesis of cyclobutanecarbaldehydes by [2+2] photocycloaddition. Angew. Chem. Int. Ed. 59, 9659–9668 (2020).
Müller, C., Bauer, A. & Bach, T. Light-driven enantioselective organocatalysis. Angew. Chem. Int. Ed. 48, 6640–6642 (2009).
Maturi, M. M. et al. Intramolecular [2+2] photocycloaddition of 3- and 4-(but-3-enyl)oxyquinolones: influence of the alkene substitution pattern, photophysical studies, and enantioselective catalysis by a chiral sensitizer. Chem. Eur. J. 19, 7461–7472 (2013).
Sorigué, D. et al. An algal photoenzyme converts fatty acids to hydrocarbons. Science 357, 903–907 (2017).
Heyes, D. J. et al. Photochemical mechanism of light-driven fatty acid photodecarboxylase. ACS Catal. 10, 6691–6696 (2020).
Heyes, D. J. et al. Photocatalysis as the ‘master switch’ of photomorphogenesis in early plant development. Nat. Plants 7, 268–276 (2021).
Tan, C. et al. The molecular origin of high DNA-repair efficiency by photolyase. Nat. Commun. 6, 7302 (2015).
Liu, X. et al. A genetically encoded photosensitizer protein facilitates the rational design of a miniature photocatalytic CO2-reducing enzyme. Nat. Chem. 10, 1201–1206 (2018).
Black, M. J. et al. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent ‘ene’-reductases. Nat. Chem. 12, 71–75 (2020).
Gao, X., Turek-Herman, J. R., Choi, Y. J., Cohen, R. D. & Hyster, T. K. Photoenzymatic synthesis of α-tertiary amines by engineered flavin-dependent “ene”-reductases. J. Am. Chem. Soc. 143, 19643–19647 (2021).
Emmanuel, M. A., Greenberg, N. R., Oblinsky, D. G. & Hyster, T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540, 414–417 (2016).
Huang, X. et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition. Nat. Catal. 5, 586–593 (2022).
Huang, X. et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 584, 69–74 (2020).
Bulina, M. E. et al. A genetically encoded photosensitizer. Nat. Biotechnol. 24, 95–99 (2006).
Fu, Y. et al. Biocatalytic cross-coupling of aryl halides with a genetically engineered photosensitizer artificial dehalogenase. J. Am. Chem. Soc. 143, 617–622 (2021).
Kang, F. et al. Rational design of a miniature photocatalytic CO2-reducing enzyme. ACS Catal. 11, 5628–5635 (2021).
Chin, J. W., Martin, A. B., King, D. S., Wang, L. & Schultz, P. G. Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proc. Natl Acad. Sci. USA 99, 11020–11024 (2002).
Godfrey, T. S., Hilpern, J. W. & Porter, G. Triplet-triplet absorption spectra of benzophenone and its derivatives. Chem. Phys. Lett. 1, 490–492 (1967).
Daub, M. E. et al. Enantioselective [2+2] cycloadditions of cinnamate esters: generalizing Lewis acid catalysis of triplet energy transfer. J. Am. Chem. Soc. 141, 9543–9547 (2019).
Lovelock, S. L. et al. The road to fully programmable protein catalysis. Nature 606, 49–58 (2022).
Tröster, A., Alonso, R., Bauer, A. & Bach, T. Enantioselective intermolecular [2 + 2] photocycloaddition reactions of 2(1H)-quinolones induced by visible light irradiation. J. Am. Chem. Soc. 138, 7808–7811 (2016).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Joosten, R. P., Joosten, K., Cohen, S. X., Vriend, G. & Perrakis, A. Automatic rebuilding and optimization of crystallographic structures in the Protein Data Bank. Bioinformatics 27, 3392–3398 (2011).
Acknowledgements
We acknowledge the European Research Council (ERC Starting Grant no. 757991 to A.P.G.) and the Biotechnology and Biological Sciences Research Council (David Phillips Fellowship BB/M027023/1 to A.P.G. and Transition Award BB/W014483/1). J.S.T. was supported by an integrated catalysis Doctoral Training Programme (EP/023755/1). R.C. was supported by a BBSRC Flexible Talent Mobility Account Award (BB/S507969/1). We are grateful to Diamond Light Source for time on beamline I03 under proposal MX24447, to the Manchester SYNBIOCHEM Centre (BB/M017702/1), the Future Biomanufacturing Hub (EP/S01778X/1) and the Henry Royce Institute for Advanced Materials (financed through EPSRC grant nos. EP/R00661X/1, EP/S019367/1, EP/P025021/1 and EP/P025498/1) for access to their facilities and to M. Dunstan (Manchester Institute of Biotechnology) for guidance on automating directed-evolution workflows. We thank R. Spiess and R. Sung (Manchester Institute of Biotechnology) for acquiring protein mass spectra and for assistance with UPLC method development and Reach Separations (Nottingham) for supplying individual enantiomers of the product 1a. We thank S. Lovelock and R. Jamagne for their assistance in preparing DNA constructs and modified proteins. We are grateful to GlaxoSmithKline for access to their facilities and to J. Hosford and L. J. Edwards (GlaxoSmithKline, Stevenage) for helpful discussions. We are also grateful to D. Leonori for helpful discussions throughout the project.
Author information
Authors and Affiliations
Contributions
J.S.T. carried out organic synthesis and substrate profiling of EnT variants. R.C. carried out molecular biology, directed-evolution experiments and protein crystallization. J.S.T. and R.C. carried out protein production, assay development and photochemical assays. F.J.H. and C.W.L. interpreted, analysed and presented structural data. R.O., D.J.H., M.J.B.B. and D.E.F. contributed to experimental design and data analysis. A.P.G., J.S.T., R.C., F.J.H. and R.O. discussed the results and participated in writing the manuscript. All authors provided input throughout project progression. A.P.G. initiated and directed the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 BpA positioning in the DA_20_00 scaffold.
a, Positions within DA_20_00 selected to incorporate the photosensitizer BpA are shown as green CPK spheres at the C-alpha. b, Bar chart showing reaction conversions and selectivities achieved by DA_20_00 variants with BpA installed at the specified positions. Reaction conditions: 15 μM catalyst, 400 μM 1, 30 min irradiation (10 s on/off pulse) at 365 nm, 4 °C, 100 μl PBS (pH 7.4) with 5% DMSO as a cosolvent in a 96-well plate. e.e. is given for the major product, (-)-1a. Error bars represent the standard deviation of measurements made in triplicate.
Extended Data Fig. 2 Summary of alanine scan of EnT1.0.
a, Positions within EnT1.0 selected for the alanine scan of the active site are shown as atom-coloured sticks with pink carbon atoms. BpA173 is shown in green CPK spheres. b, Bar chart showing reaction conversions and regioselectivities achieved by EnT1.0 variants. Reaction conditions: 15 μM catalyst, 400 μM 1, 30 min irradiation (10 s on/off pulse) at 365 nm, 4 °C, 100 μl PBS (pH 7.4) with 5% DMSO as a cosolvent in a 96-well plate. Error bars represent the standard deviation of measurements made in triplicate.
Extended Data Fig. 3 Directed evolution of an efficient and enantioselective photoenzyme.
Schematic showing the trajectory from DA_20_00 to EnT1.3 and EnT1.3 Y121F S271C. Mutations introduced are represented as CPK spheres at the C-alpha. The original design DA_20_00 contains 13 mutations built into a six-bladed β-propeller scaffold (PDB 1E1A; a diisopropylfluorophosphatase from Loligo vulgaris)13. Incorporation of the photosensitizer BpA (green atom-coloured sticks and semitransparent CPK spheres) at position A173 provided EnT1.0. An alanine scan of EnT1.0 highlighted one variant with increased activity, providing EnT1.1. Two rounds of evolution afforded EnT1.3, which contains six mutations when compared with the original design DA_20_00. EnT1.3 was further evolved towards substrate 13, resulting in EnT1.3 Y121F S271C. Library generation method, positions targeted, the number of clones evaluated, beneficial mutations and the most improved variant for each round are given in the table.
Extended Data Fig. 4 Reaction time courses of EnT1.0 and EnT1.3.
Reaction conditions: 15 μM catalyst, 400 μM 1, 10 s on/off pulse at 365 nm, 4 °C, 1 ml PBS (pH 7.4) with 5% DMSO as a cosolvent in 2-ml glass vials. At each time point, 100 μl of reaction mix was quenched with one volume of MeCN and analysed by UPLC. Reaction conversion of 1 to 1a and 1b is given over 5 min. The gradients of a linear fit to the data are given for each variant. A table providing conversion data is given in Supplementary Table S2. Error bars represent the standard deviation of measurements made in triplicate (error bars are not shown when they are smaller than the data-point marker).
Extended Data Fig. 5 Photodamage of EnT1.0 and EnT1.3.
To investigate the effects of photodamage, aliquots of 30 μM enzyme in PBS (pH 7.4) were pre-irradiated for 90 min (10 s on/off pulse at 365 nm) at 4 °C, before performing reactions. Control aliquots of enzyme were incubated in the dark at 4 °C. a, Bar chart showing the conversion of 1 to 1a and 1b achieved by variants DA_20_00, EnT1.0 and EnT1.3 that had been pre-irradiated with light (+) and had been incubated in the dark (−). Reaction conditions: 15 μM catalyst, 400 μM 1, 30 min irradiation (10 s on/off pulse) at 365 nm, 4 °C, PBS (pH 7.4) with 5% DMSO as a cosolvent. Error bars represent the standard deviation of measurements made in triplicate. b, SDS-PAGE analysis of protein variants (DA_20_00, EnT1.0, EnT1.3) that had been pre-irradiated with light (+) and had been incubated in the dark (−).
Extended Data Fig. 6 Temperature dependence of EnT1.3.
Reaction conditions: 2.25 μM catalyst (7.5 mol%), 300 μM 1, 10 s on/off pulse at 365 nm, in PBS (pH 7.4) with 5% DMSO as a cosolvent at a specified temperature. A time course providing the conversion of 1 to 1a and 1b at 4 °C (blue circles) and room temperature (red circles). A negative control containing no catalyst is shown at 4 °C and room temperature (blue squares and red squares, respectively). Error bars represent the standard deviation of measurements made in triplicate.
Extended Data Fig. 7 Total turnover numbers achieved by EnT1.3.
Reaction conditions: 800 μM 1, 10 s on/off pulse at 365 nm, at 4 °C, in 1 ml PBS (pH 7.4) with 10% DMSO as a cosolvent, at a specified catalyst loading. A time course providing the reaction conversion of 1 to 1a and 1b at 0.2 mol% and 1.5 mol% catalyst loading (1.6 μM (red) and 12 μM (blue), respectively). A background correction has been applied to both curves, corresponding to less than 10% of turnovers. Error bars represent the standard deviation of measurements made in triplicate (error bars are not shown when they are smaller than the data-point marker).
Extended Data Fig. 8 Evolution trajectory.
Reaction conditions: 10 μM catalyst, 400 μM 1, 30 min irradiation (10 s on/off pulse) at 365 nm, 4 °C, PBS (pH 7.4) with 5% DMSO as a cosolvent in glass vials. a, Bar chart showing conversion of 1 to 1a and 1b and e.e. for the major product, (-)-1a, achieved by selected variants along the evolutionary trajectory. Error bars represent the standard deviation of measurements made in triplicate (error bars are not shown when they are smaller than the data-point marker). b, Table providing conversion and selectivity data for Extended Data Fig. 8a.
Extended Data Fig. 9 Knockout variants of EnT1.3.
a, Positions in EnT1.3 selected for a mutational scan are shown as atom-coloured sticks, with carbon grey. BpA is shown as atom-coloured sticks, with carbon green. b, Reaction conditions: 7.5 μM catalyst, 400 μM 1, 15 min irradiation (10 s on/off pulse) at 365 nm, 4 °C, 100 μl PBS (pH 7.4) with 5% DMSO as a cosolvent in 96-well plates. Bar chart showing conversions and e.e. for the major product, (-)-1a, achieved by EnT1.3 variants. Error bars represent the standard deviation of measurements made in triplicate. c, Table providing conversion and selectivity data for Extended Data Fig. 9b.
Extended Data Fig. 10 Comparison of apo-EnT1.3 (PDB: 7ZP6) and product-bound EnT1.3ΔC310-314 (PDB: 7ZP7).
a, EnT1.3 (shown as a grey cartoon) has a hydrogen-bonding network formed of E225, R196 and D149 (shown as dashed black lines). The side chains of key residues are shown as atom-coloured sticks with grey carbons. BpA173 is shown as atom-coloured sticks and semitransparent CPK spheres with green carbons. b, The structure of EnT1.3ΔC310-314 (shown as a blue cartoon) in complex with the product (-)-1a (shown as atom-coloured sticks with purple carbons). The side chains of key residues are shown as atom-coloured sticks with blue carbons. BpA173 is shown as atom-coloured sticks and semitransparent CPK spheres with green carbons. When in complex with product, R196 lies in a different conformation to that shown in the structure of apo-EnT1.3, instead forming a hydrogen bond with Q195. Q195 and Y121 form close hydrogen bonds to (-)-1a.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Supplementary Tables 1–11, a list of protein and DNA sequences and NMR spectra data.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trimble, J.S., Crawshaw, R., Hardy, F.J. et al. A designed photoenzyme for enantioselective [2+2] cycloadditions. Nature 611, 709–714 (2022). https://doi.org/10.1038/s41586-022-05335-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05335-3
This article is cited by
-
Remote stereocontrol with azaarenes via enzymatic hydrogen atom transfer
Nature Chemistry (2024)
-
Accessing ladder-shape azetidine-fused indoline pentacycles through intermolecular regiodivergent aza-Paternò–Büchi reactions
Nature Communications (2024)
-
A light-driven enzymatic enantioselective radical acylation
Nature (2024)
-
A non-canonical nucleophile unlocks a new mechanistic pathway in a designed enzyme
Nature Communications (2024)
-
Modular and diverse synthesis of amino acids via asymmetric decarboxylative protonation of aminomalonic acids
Nature Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.