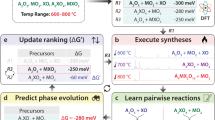
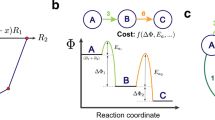
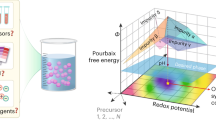
Abstract
Advancements in many modern technologies rely on the continuous need for materials discovery. However, the design of synthesis routes leading to new and targeted solid-state materials requires understanding of reactivity patterns1,2,3. Advances in synthesis science are necessary to increase efficiency and accelerate materials discovery4,5,6,7,8,9,10. We present a highly effective methodology for the rational discovery of materials using high-temperature solutions or fluxes having tunable solubility. This methodology facilitates product selection by projecting the free-energy landscape into real synthetic variables: temperature and flux ratio. We demonstrate the effectiveness of this technique by synthesizing compounds in the chalcogenide system of A(Ba)-Cu-Q(O) (Q = S or Se; A = Na, K or Rb) using mixed AOH/AX (A = Li, Na, K or Rb; X = Cl or I) fluxes. We present 30 unreported compounds or compositions, including more than ten unique structural types, by systematically varying the temperature and flux ratios without requiring changing the proportions of starting materials. Also, we found that the structural dimensionality of the compounds decreases with increasing reactant solubility and temperature. This methodology serves as an effective general strategy for the rational discovery of inorganic solids.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, with deposition numbers 2184424–2184451, corresponding to compounds shown in Table 1: I (Na4+xOCu4Se4, CSD 2184424), II (Na10+xO2Cu11+xSe10, CSD 2184425), III (Na5+xOCu8Se6, CSD 2184427), IV (Na4+xOCu4S4, CSD 2184435), V (Na10+xO2Cu11+xS10, CSD 2184429), VI (Na5+xOCu8S6, CSD 2184433), VIII (K4+xOCu4Se4, CSD 2184438), IX (NaCu3S2, CSD 2184426), X (NaCu3Se2, CSD 2184428), XII (Na(Cu0.6Li0.4)S, CSD 2184430), XIII (Na(Cu0.6Li0.4)Se, CSD 2184431), XIV (BaCu1.4Li0.6S2, CSD 2184436), XV ((Ba0.44Rb0.56)Cu2Se2, CSD 2184441), XVI (Na3BaCu7S6, CSD 2184432), XVII (K3BaCu7S6, CSD 2184434), XVIII (Ba2Cu2Na1.3O1.1S3, CSD 2184442), XIX (Ba4.5Cu6.7Na1.7O4S6, CSD 2184439), XX (Ba2−xCu5.5OSe4, CSD 2184437), XXI (Ba2Cu0.8O2Cu2Se2, CSD 2184443), XXII (Ba2Na0.55O2Cu2Se2, CSD 2184440), XXIII ((Ba1.63K0.37)O2Cu2Se2, CSD 2184445), XXIV (Ba2Cu0.8O3CuS, CSD 2184450), XXV (KCu5Se3, CSD 2184444), XXVI (RbCu7−xSe4, CSD 2184448), XXVII (Ba4Rb6Cu12Se13, CSD 2184447), XXVIII (Na3Cu4Se4, CSD 2184446), XXIX (BaK2Cu4S4−xSex, CSD 2184451), XXX (BaK2Cu8S6, CSD 2184449). Copies of the data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures/. Source data for Extended Data Fig. 3 is provided within this paper. Data are also available on request. Source data are provided with this paper.
References
Kanatzidis, M. G. Discovery-synthesis, design, and prediction of chalcogenide phases. Inorg. Chem. 56, 3158–3173 (2017).
Shoemaker, D. P. et al. Understanding fluxes as media for directed synthesis: in situ local structure of molten potassium polysulfides. J. Am. Chem. Soc. 134, 9456–9463 (2012).
Haynes, A. S., Stoumpos, C. C., Chen, H., Chica, D. & Kanatzidis, M. G. Panoramic synthesis as an effective materials discovery tool: the system Cs/Sn/P/Se as a test case. J. Am. Chem. Soc. 139, 10814–10821 (2017).
Nunn, W. et al. Novel synthesis approach for “stubborn” metals and metal oxides. Proc. Natl Acad. Sci. USA 118, e2105713118 (2021).
Sanchez-Lengeling, B. & Aspuru-Guzik, A. Inverse molecular design using machine learning: generative models for matter engineering. Science 361, 360–365 (2018).
Schmidt, J., Marques, M. R. G., Botti, S. & Marques, M. A. L. Recent advances and applications of machine learning in solid-state materials science. NPJ Comput. Mater. 5, 83 (2019).
Oganov, A. R., Pickard, C. J., Zhu, Q. & Needs, R. J. Structure prediction drives materials discovery. Nat. Rev. Mater. 4, 331–348 (2019).
Alberi, K. et al. The 2019 materials by design roadmap. J. Phys. D Appl. Phys. 52, 013001 (2018).
Tang, F., Po, H. C., Vishwanath, A. & Wan, X. Efficient topological materials discovery using symmetry indicators. Nat. Phys. 15, 470–476 (2019).
Tabor, D. P. et al. Accelerating the discovery of materials for clean energy in the era of smart automation. Nat. Rev. Mater. 3, 5–20 (2018).
Corbett, J. D. Exploratory synthesis in the solid state. Endless wonders. Inorg. Chem. 39, 5178–5191 (2000).
Arachchige, I. U. et al. Mercouri G. Kanatzidis: excellence and innovations in inorganic and solid-state chemistry. Inorg. Chem. 56, 7582–7597 (2017).
Kovnir, K. Predictive synthesis. Chem. Mater. 33, 4835–4841 (2021).
Chiotti, P. & Markuszewski, R. Binary systems sodium sulfide-sodium hydroxide and sodium carbonate-sodium hydroxide. J. Chem. Eng. Data 30, 197–201 (1985).
Seefuth, R. N. & Sharma, R. A. Solubility of Li2 S in LiCl ‐ KCl melts. J. Electrochem. Soc. 135, 796 (1988).
Androulakis, J. et al. Dimensional reduction: a design tool for new radiation detection materials. Adv. Mater. 23, 4163–4167 (2011).
Ganglberger, E. Die Kristallstruktur von Nb5Cu4Si4. Monatsh. Chem. Chem. Mon. 99, 549–556 (1968).
Zhou, X. et al. New compounds and phase selection of nickel sulfides via oxidation state control in molten hydroxides. J. Am. Chem. Soc. 143, 13646–13654 (2021).
Friedrich, A., Kunz, M., Miletich, R. & Pattison, P. High-pressure behavior of Ba(OH)2 phase transitions and bulk modulus. Phys. Rev. B 66, 214103 (2002).
Zhang, X., Hogan, T., Kannewurf, C. R. & Kanatzidis, M. G. Sulfur p-band hole generation in β-BaCu2S2. Synthesis of metallic KxBa1−xCu2S2 from molten mixed K·Ba polysulfide salts. J. Alloys Compd. 236, 1–5 (1996).
Li, W. et al. Synthesis, structure, and properties of the layered oxyselenide Ba2CuO2Cu2Se2. Inorg. Chem. 57, 5108–5113 (2018).
Lux, H., Kuhn, R. & Niedermaier, T. Reaktionen und Gleichgewichte in Alkalihydroxydschmelzen. III. Peroxydgleichgewichte. Z. Anorg. Allg. Chem. 298, 285–301 (1959).
Flood, H. & Förland, T. The acidic and basic properties of oxides. Acta Chem. Scand. 1, 592–606 (1947).
Pöhls, J.-H., Heyberger, M. & Mar, A. Comparison of computational and experimental inorganic crystal structures. J. Solid State Chem. 290, 121557 (2020).
Jansen, M. A concept for synthesis planning in solid-state chemistry. Angew. Chem. Int. Edn 41, 3746–3766 (2002).
Jansen, M. & Schön, J. C. “Design” in chemical synthesis—an illusion? Angew. Chem. Int. Edn 45, 3406–3412 (2006).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Albrecht, R. & Ruck, M. Chalcogenides by reduction of their dioxides in ultra-alkaline media. Angew. Chem. Int. Edn 60, 22570–22577 (2021).
Bugaris, D. E., Smith, M. D. & zur Loye, H.-C. Hydroflux crystal growth of platinum group metal hydroxides: Sr6NaPd2(OH)17, Li2Pt(OH)6, Na2Pt(OH)6, Sr2Pt(OH)8, and Ba2Pt(OH)8. Inorg. Chem. 52, 3836–3844 (2013).
Chance, W. M., Bugaris, D. E., Sefat, A. S. & zur Loye, H.-C. Crystal growth of new hexahydroxometallates using a hydroflux. Inorg. Chem. 52, 11723–11733 (2013).
Klepov, V. V., Juillerat, C. A., Pace, K. A., Morrison, G. & zur Loye, H.-C. “Soft” alkali bromide and iodide fluxes for crystal growth. Front. Chem. 8, 518 (2020).
Mugavero III, S. J., Gemmill, W. R., Roof, I. P. & zur Loye, H.-C. Materials discovery by crystal growth: lanthanide metal containing oxides of the platinum group metals (Ru, Os, Ir, Rh, Pd, Pt) from molten alkali metal hydroxides. J. Solid State Chem. 182, 1950–1963 (2009).
Chica, D. G. et al. Direct thermal neutron detection by the 2D semiconductor 6LiInP2Se6. Nature 577, 346–349 (2020).
Effenberger, H. & Pertlik, F. Crystal structure of NaCu5S3. Monatsh. Chem. Chem. Mon. 116, 921–926 (1985).
Savelsberg, G. Ternäre Pnictide und Chalkogenide von Alkalimetallen und IB-bzw. IIB-Elementen/On ternary pnictides and chalkogenides of alkaline metals and IB-resp. II B-elements. Z. Naturforsch. B 33, 370–373 (1978).
Li, J., Guo, H.-Y., Zhang, X. & Kanatzidis, M. G. CsAg5Te3: a new metal-rich telluride with a unique tunnel structure. J. Alloys Compd. 218, 1–4 (1995).
Rettie, A. J. E. et al. Copper vacancies and heavy holes in the two-dimensional semiconductor KCu3−xSe2. Chem. Mater. 29, 6114–6121 (2017).
Näther, C., Röhnert, D. & Bensch, W. Synthesis, crystal structure and low-temperature X-ray investigations of K3Cu8Se6. Eur. J. Solid State Inorg. Chem. 35, 565–577 (1998).
Tiedje, O. et al. Bridging from ThCr2Si2-type materials to hexagonal dichalcogenides: an ab initio and experimental study of KCu2Se2. Phys. Rev. B 67, 134105 (2003).
Burschka, C. & Bronger, W. KCu3S2, ein neues Thiocuprat/KCu3S2, a new thiocuprate. Z. Naturforsch. B 32, 11–14 (1977).
Fuhr, O., Dehnen, S. & Fenske, D. Chalcogenide clusters of copper and silver from silylated chalcogenide sources. Chem. Soc. Rev. 42, 1871–1906 (2013).
Shoemaker, D. P. et al. In situ studies of a platform for metastable inorganic crystal growth and materials discovery. Proc. Natl Acad. Sci. USA 111, 10922–10927 (2014).
Schils, H. & Bronger, W. Ternäre Selenide des Kupfers. Z. Anorg. Allg. Chem. 456, 187–193 (1979).
Acknowledgements
This work was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division. Work carried out at the Center for Nanoscale Materials (SEM, ACAT and Carbon high-performance computing cluster), a US Department of Energy (DOE) Office of Science User Facility, was supported by the US DOE Office of Basic Energy Sciences under contract no. DE-AC02-06CH11357. The computational work is supported by the US DOE Office of Science Scientific User Facilities AI/ML project titled 'A digital twin for spatiotemporally resolved experiments.' M.K.Y.C. acknowledges support from the BES SUFD Early Career award. Work at the beamlines 15-IDD and 17-BM at the Advanced Photon Source (APS) at Argonne National Laboratory was supported by the US DOE, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-06CH11357. NSF’s ChemMatCARS Sector 15 is supported by the Divisions of Chemistry (CHE) and Materials Research (DMR), National Science Foundation, under grant no. NSF/CHE-1834750.
Author information
Authors and Affiliations
Contributions
The work was conceived by X.Z., D.-Y.C. and M.G.K., with input from all authors. X.Z. carried out the synthesis, lab X-ray diffraction and elemental analysis. X.Z. and W.X. collected and analysed in situ synchrotron diffraction data. V.-S.-C.K., L.W. and M.K.Y.C. performed first-principle calculations. X.Z., T.C. and Y.-S.C. collected and analysed single-crystal diffraction data. L.Y. and J.W. collected and analysed electron energy loss spectroscopy spectra. D.-Y.C. and M.G.K. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Hans Conrad zur Loye and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Reaction pathways in the (K, Li)OH/KI flux.
a, XXV (KCu5Se3). b, α-KCu3Se2. c, K3Cu8Se6. d, KCu2Se2. e, K4+xOCu4Q4. f, XI (KCu3S2). g, α-KCu3S2. Compounds from Table 1 are shown as VII (K4+xOCu4S4), VIII (K4+xOCu4Se4), XI (KCu3S2) and XXV (KCu5Se3). The crystal structures VII and VIII are identical to that of I (Na4+xOCu4Se4) and XI is isostructural with IX (KCu3S2). Purple, blue, red, light yellow and light green spheres represent K, Cu, O, S and Se atoms, respectively.
Extended Data Fig. 2 Reaction pathways in the (Rb, Li)OH/RbI flux.
a, RbCu4Se3. b, XXVI (RbCu7−xSe4). c, Rb3Cu8Se6. Compounds from Table 1 are shown as XXVI (RbCu7−xSe4). Pink, blue and light green spheres represent Rb, Cu and Se atoms, respectively.
Extended Data Fig. 3 Panoramic synthesis.
In situ synchrotron powder X-ray diffraction patterns of reactions collected in the mixed flux of NaOH/NaI for [OH] = 0.60, Q = Se (a), [OH] = 0.80, Q = Se (b), [OH] = 0.80, Q = Se, Na/Li = 1 (c) and [OH] = 0.65, Q = S with addition of BaO (d). Their respective temperature profiles are shown in Fig. S3. G marked with the purple box in d is probably several different unknown phases with overlapping Bragg peaks.
Supplementary information
Supplementary Information
This file contains further experimental details and Supplementary Figures 1–13.
CIF files for Supplementary Data 1–4
See Supplementary Information for TOC.
Source data
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, X., Kolluru, V.S.C., Xu, W. et al. Discovery of chalcogenides structures and compositions using mixed fluxes. Nature 612, 72–77 (2022). https://doi.org/10.1038/s41586-022-05307-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05307-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.