Abstract
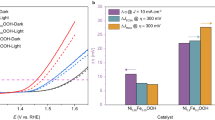
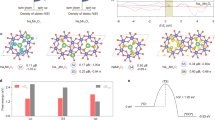
Realizing an efficient electron transfer process in the oxygen evolution reaction by modifying the electronic states around the Fermi level is crucial in developing high-performing and robust electrocatalysts1,2,3. Typically, electron transfer proceeds solely through either a metal redox chemistry (an adsorbate evolution mechanism (AEM), with metal bands around the Fermi level) or an oxygen redox chemistry (a lattice oxygen oxidation mechanism (LOM), with oxygen bands around the Fermi level), without the concurrent occurrence of both metal and oxygen redox chemistries in the same electron transfer pathway1,2,3,4,5,6,7,8,9,10,11,12,13,14,15. Here we report an electron transfer mechanism that involves a switchable metal and oxygen redox chemistry in nickel-oxyhydroxide-based materials with light as the trigger. In contrast to the traditional AEM and LOM, the proposed light-triggered coupled oxygen evolution mechanism requires the unit cell to undergo reversible geometric conversion between octahedron (NiO6) and square planar (NiO4) to achieve electronic states (around the Fermi level) with alternative metal and oxygen characters throughout the oxygen evolution process. Utilizing this electron transfer pathway can bypass the potential limiting steps, that is, oxygen–oxygen bonding in AEM and deprotonation in LOM1,2,3,4,5,8. As a result, the electrocatalysts that operate through this route show superior activity compared with previously reported electrocatalysts. Thus, it is expected that the proposed light-triggered coupled oxygen evolution mechanism adds a layer of understanding to the oxygen evolution research scene.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting of the finding of this study are included within the paper and its Supplementary Information files and are available from the corresponding authors on request.
References
Grimaud, A., Hong, W. T., Shao-Horn, Y. & Tarascon, J. M. Anionic redox progresses for electrochemical devices. Nat. Mater. 15, 121–126 (2016).
Huang, Z. F. et al. Chemical and structural origin of lattice oxygen oxidation in Co–Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Ener. 4, 329–338 (2019).
Song, J. et al. A review on fundamental for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 49, 2196 (2020).
Grimaud, A. et al. Activating lattice oxygen redox reaction in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
Mefford, J. T. et al. Water electrolysis on La1−xSrxCoO3−δ perovskite electrocatalysts. Nat. Commun. 7, 11503 (2017).
Pan, Y. et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun. 11, 2002 (2020).
Hibbert, D. B. & Churchill, C. R. Kinetics of the electrochemical evolution of isotopically enriched gases part 2.—18O16O evolution on NiCo2O4 and LixCo3−xO4 in alkaline solution. J. Chem. Soc. Faraday Trans. 80, 1965–1975 (1984).
Wang, X. et al. Strain stabilized nickel hydroxide nanoribbons for efficient water splitting. Energy Environ. Sci. 13, 229–237 (2020).
Nong, H. N. et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature 587, 408–413 (2020).
Rao, R. et al. Towards identifying the active sites on RuO2 (110) in catalyzing oxygen evolution. Energy Environ. Sci. 10, 2626–2637 (2017).
Halck, N., Petrykin, V., Krtil, P. & Rossmeisl, J. Beyond the volcano limitations in electrocatalysis-oxygen evolution reaction. Phys. Chem. Chem. Phys. 16, 13682–13688 (2014).
Craig, M. et al. Universal scaling relations for the rational design of molecular water oxidation catalysts with near-zero overpotential. Nat. Commun. 10, 4993 (2019).
Retuerto, M. et al. Role of lattice oxygen content and Ni geometry in the oxygen evolution activity of the Ba-Ni-O system. J. Power Sources 404, 56–63 (2018).
Garces-Pineda, F., Blasco-Ahicart, M., Nieto-Castro, D., Lopez, N. & Galan-Mascaros, J. Direct magnetic enhancement of electrocatalytic water oxidation in alkaline media. Nat. Energy 4, 519–525 (2019).
Gracia, J. Spin dependent interactions catalyse the oxygen electrochemistry. Phys. Chem. Chem. Phys. 19, 20451–20456 (2017).
Wang, X. et al. Materializing efficient methanol oxidation via electron delocalization in nickel hydroxide nanoribbon. Nat. Commun. 11, 4647 (2020).
Colburn, A. W., Levey, K. J., Ohare, D. & Macpherson, J. V. Lifting the lid on the potentiostat: a beginner’s guide to understanding electrochemical circuitry and practical operation. Phys. Chem. Chem. Phys. 23, 8100–8117 (2021).
Dionigi, F. & Strasser, P. NiFe-based (oxy)hydroxide catalysts for oxygen evolution reaction in non-acidic electrolytes. Adv. Energy Mater. 6, 1600621 (2016).
Wei, C. et al. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 31, 1806296 (2019).
Morales, D. M. & Risch, M. Seven steps to reliable cyclic voltammetry measurements for the determination of double layer capacitance. J. Phys. Energy 3, 034013 (2021).
Formal, F. et al. Back electron-hole recombination in hematite photoanodes for water splitting. J. Am. Chem. Soc. 136, 2564–2574 (2014).
Krysiak, O., Cichowicz, G., Conzuelo, F., Cyranski, M. & Augustynski, J. Ni–Fe–Cr-oxides: an efficient catalyst activated by visible light for the oxygen evolution reaction. Z. Phys. Chem. 234, 633–643 (2020).
Suntivich, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011).
Bediako, D. K. et al. Structure activity correlations in a nickel-borate oxygen evolution catalyst. J. Am. Chem. Soc. 134, 6801–6809 (2012).
McBreen, J. et al. In situ time-resolved X-ray absorption near edge structure study of the nickel oxide electrode. J. Phys. Chem. 93, 6308–6311 (1989).
Friebel, D. et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015).
Ohtsu, H. & Tanaka, K. Equilibrium of low- and high-spin states of Ni(II) complexes controlled by the donor ability of bidentate ligands. Inorg. Chem. 43, 3024–3030 (2004).
Nast, R. Coordination chemistry of metal alkynyl compounds. Coord. Chem. Rev. 47, 125–164 (1982).
Tao, Z. et al. The nature of photoinduced phase transition and metastable states in vanadium dioxide. Sci. Rep. 6, 38514 (2016).
Yang, C., Fontaine, O., Tarascon, J. & Grimaud, A. Chemical recognition of active oxygen species on the surface of oxygen evolution reaction electrocatalysts. Angew. Chem. Int. Edn 129, 8778–8782 (2017).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Köhler, L., Abrishami, M., Raddatis, V., Geppert, J. & Risch, M. Mechanistic parameters of electrocatalytic water oxidation on LiMn2O4 in comparison to natural photosynthesis. ChemSusChem 10, 4479–4490 (2017).
Zhang, Q. & Asthagiri, A. Solvation effects on DFT predictions of ORR activity on metal surfaces. Catal. Today 323, 35–43 (2019).
Trzesniewski, B. J. et al. In situ observation of active oxygen species in Fe-containing Ni-based oxygen evolution catalysts: the effect of pH on electrochemical activity. J. Am. Chem. Soc. 137, 15112–15121 (2015).
Hao, Y. et al. Recognition of surface oxygen intermediates on NiFe oxyhydroxide oxygen-evolving catalysts by homogeneous oxidation reactivity. J. Am. Chem. Soc. 143, 1493–1502 (2021).
Roy, C. et al. Impact of nanoparticles size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 1, 820–829 (2018).
Zhang, F. et al. Decoupled redox catalytic hydrogen production with a robust electrolyte-Borne electron and proton carrier. J. Am. Chem. Soc. 143, 223–231 (2021).
Acknowledgements
We thank H. Wang and C. Wang for their help with figure design and A. Stefan for his advice on simulation. This work is financially supported by Singapore Ministry of Education (MOE) Tier 1 R284000226114 and MOE Tier 2 (MOE2018-T2-1-149), Agency for Science, Technology and Research (A*STAR) of Singapore. This research is also supported by A*STAR, grant number 152-70-00017, and computational resources were provided by National Supercomputing Centre Singapore (NSCC) and A*STAR Computational Resource Centre, Singapore (A*CRC). This project was partly supported by the Science and Engineering Research Council (SERC) of A*STAR of Singapore. This research is also supported by the Guangxi Bagui Scholar Foundation, Guilin Lijiang Scholar Foundation, Science and Technology Development Project of Guilin (20210216-1).
Author information
Authors and Affiliations
Contributions
X.W., S.X. and J.X. conceived the idea. X.W. and W.S.V.L. performed synthesis and electrochemical measurement of the samples. X.W., W.S.V.L., H.Z., Q.W., H.W. and Z.W. were responsible for the analysis of electrochemical results. S.X., Y.D. and A.B. were responsible for the XAFS characterization. Z.G.Y., P.H. and Y.-W.Z. carried out DFT simulations. J.X. is in charge of the overall project and preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Marcel Risch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Materials and Methods, Discussion, Figs. 1–41, Tables 1–6 and References.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Xi, S., Huang, P. et al. Pivotal role of reversible NiO6 geometric conversion in oxygen evolution. Nature 611, 702–708 (2022). https://doi.org/10.1038/s41586-022-05296-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05296-7
This article is cited by
-
High-spin Co3+ in cobalt oxyhydroxide for efficient water oxidation
Nature Communications (2024)
-
Tensile straining of iridium sites in manganese oxides for proton-exchange membrane water electrolysers
Nature Communications (2024)
-
Potential and electric double-layer effect in electrocatalytic urea synthesis
Nature Communications (2024)
-
Recent progress of cobalt-based electrocatalysts for water splitting: Electron modulation, surface reconstitution, and applications
Nano Research (2024)
-
Perspective for OER electrocatalysts: Lattice engineering of clay-like frameworks with near-surface cluster active sites
Science China Technological Sciences (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.