Abstract
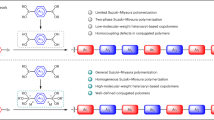
Conducting polymers (CPs) with high conductivity and solution processability have made great advances since the pioneering work on doped polyacetylene1,2,3, thus creating the new field of ‘organic synthetic metals,4. Various high-performance CPs have been realized, which enable the applications of several organic electronic devices5,6. Nevertheless, most CPs exhibit hole-dominant (p-type) transport behaviour7,8, whereas the development of n-type analogues lags far behind and only a few exhibit metallic state, typically limited by low doping efficiency and ambient instability. Here we present a facilely synthesized highly conductive n-type polymer poly(benzodifurandione) (PBFDO). The reaction combines oxidative polymerization and in situ reductive n-doping, greatly increasing the doping efficiency, and a doping level of almost 0.9 charges per repeating unit can be achieved. The resultant polymer exhibits a breakthrough conductivity of more than 2,000 S cm−1 with excellent stability and an unexpected solution processability without extra side chains or surfactants. Furthermore, detailed investigations on PBFDO show coherent charge-transport properties and existence of metallic state. The benchmark performances in electrochemical transistors and thermoelectric generators are further demonstrated, thus paving the way for application of the n-type CPs in organic electronics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the paper and its Supplementary Information file, and related source data are available from the corresponding author on reasonable request.
References
Shirakawa, H., Louis, E. J., MacDiarmid, A. G., Chiang, C. K. & Heeger, A. J. Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 16, 578–580 (1977).
Basescu, N. et al. High electrical conductivity in doped polyacetylene. Nature 327, 403–405 (1987).
MacDiarmid, A. G. & Heeger, A. J. Organic metals and semiconductors: the chemistry of polyacetylene, (CH)x, and its derivatives. Synth. Met. 1, 101–118 (1980).
Heeger, A. J. Semiconducting and metallic polymers: the fourth generation of polymeric materials (Nobel Lecture). Angew. Chem. Int. Ed. 40, 2591–2611 (2001).
Bharti, M., Singh, A., Samanta, S. & Aswal, D. K. Conductive polymers for thermoelectric power generation. Prog. Mater. Sci. 93, 270–310 (2018).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Bhadra, S., Khastgir, D., Singha, N. K. & Lee, J. H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 34, 783–810 (2009).
Gueye, M. N., Carella, A., Faure-Vincent, J., Demadrille, R. & Simonato, J.-P. Progress in understanding structure and transport properties of PEDOT-based materials: a critical review. Prog. Mater. Sci. 108, 100616 (2020).
Lu, Y. et al. Rigid coplanar polymers for stable n-type polymer thermoelectrics. Angew. Chem. Int. Ed. 58, 11390–11394 (2019).
Yang, C.-Y. et al. A high-conductivity n-type polymeric ink for printed electronics. Nat. Commun. 12, 2354 (2021).
Lüssem, B. et al. Doped organic transistors. Chem. Rev. 116, 13714–13751 (2016).
Zhao, X. et al. High conductivity and electron-transfer validation in an n-type fluoride-anion-doped polymer for thermoelectrics in air. Adv. Mater. 29, 1606928 (2017).
Han, J. et al. Dichlorinated dithienylethene-based copolymers for air-stable n-type conductivity and thermoelectricity. Adv. Funct. Mater. 31, 2005901 (2021).
Wei, P. et al. 2-(2-Methoxyphenyl)-1,3-dimethyl-1H-benzoimidazol-3-ium iodide as a new air-stable n-type dopant for vacuum-processed organic semiconductor thin films. J. Am. Chem. Soc. 134, 3999–4002 (2012).
Li, F., Werner, A., Pfeiffer, M., Leo, K. & Liu, X. Leuco crystal violet as a dopant for n-doping of organic thin films of fullerene C60. J. Phys. Chem. B 108, 17076–17082 (2004).
Yang, C.-Y. et al. A thermally activated and highly miscible dopant for n-type organic thermoelectrics. Nat. Commun. 11, 3292 (2020).
Guo, S. et al. N-doping of organic electronic materials using air-stable organometallics. Adv. Mater. 24, 699–703 (2012).
Naab, B. D. et al. Effective solution- and vacuum-processed n-doping by dimers of benzimidazoline radicals. Adv. Mater. 26, 4268–4272 (2014).
Tang, C. G. et al. Multivalent anions as universal latent electron donors. Nature 573, 519–525 (2019).
Guha, S., Goodson, F. S., Corson, L. J. & Saha, S. Boundaries of anion/naphthalenediimide interactions: from anion–π interactions to anion-induced charge-transfer and electron-transfer phenomena. J. Am. Chem. Soc. 134, 13679–13691 (2012).
Lu, Y. et al. The critical role of dopant cations in electrical conductivity and thermoelectric performance of n-doped polymers. J. Am. Chem. Soc. 142, 15340–15348 (2020).
Liu, J. et al. Enhancing molecular n-type doping of donor–acceptor copolymers by tailoring side chains. Adv. Mater. 30, 1704630 (2018).
Kiefer, D. et al. Enhanced n-doping efficiency of a naphthalenediimide-based copolymer through polar side chains for organic thermoelectrics. ACS Energy Lett. 3, 278–285 (2018).
Guo, H. et al. Transition metal-catalysed molecular n-doping of organic semiconductors. Nature 599, 67–73 (2021).
Lu, Y. et al. Persistent conjugated backbone and disordered lamellar packing impart polymers with efficient n-doping and high conductivities. Adv. Mater. 33, 2005946 (2021).
Sun, Y. et al. Flexible n-type high-performance thermoelectric thin films of poly(nickel-ethylenetetrathiolate) prepared by an electrochemical method. Adv. Mater. 28, 3351–3358 (2016).
Guo, X. & Facchetti, A. The journey of conducting polymers from discovery to application. Nat. Mater. 19, 922–928 (2020).
Zozoulenko, I. et al. Polarons, bipolarons, and absorption spectroscopy of PEDOT. ACS Appl. Polym. Mater. 1, 83–94 (2019).
Emin, D. Optical properties of large and small polarons and bipolarons. Phys. Rev. B 48, 13691–13702 (1993).
Mueller, P. & Rocek, J. Oxidations of hydroaromatic systems. II. 2,3-Dichloro-5,6-dicyanobenzoquinone. J. Am. Chem. Soc. 94, 2716–2719 (1972).
Cheng, D. & Bao, W. Propargylation of 1,3-dicarbonyl compounds with 1,3-diarylpropynes via oxidative cross-coupling between sp3 C–H and sp3 C–H. J. Org. Chem. 73, 6881–6883 (2008).
Zhang, Y. & Li, C.-J. DDQ-mediated direct cross-dehydrogenative-coupling (CDC) between benzyl ethers and simple ketones. J. Am. Chem. Soc. 128, 4242–4243 (2006).
Li, Z. et al. C–H bond oxidation initiated Pummerer- and Knoevenagel-type reactions of benzyl sulfide and 1,3-dicarbonyl compounds. Org. Lett. 10, 803–805 (2008).
Fischer, H. The persistent radical effect: a principle for selective radical reactions and living radical polymerizations. Chem. Rev. 101, 3581–3610 (2001).
Kobayashi, Y., Yoshioka, M., Saigo, K., Hashizume, D. & Ogura, T. Hydrogen-bonding-assisted self-doping in tetrathiafulvalene (TTF) conductor. J. Am. Chem. Soc. 131, 9995–10002 (2009).
Menon, R., Yoon, C. O., Moses, D., Heeger, A. J. & Cao, Y. Transport in polyaniline near the critical regime of the metal-insulator transition. Phys. Rev. B 48, 17685–17694 (1993).
Ahlskog, M., Reghu, M. & Heeger, A. J. The temperature dependence of the conductivity in the critical regime of the metal-insulator transition in conducting polymers. J. Phys. Condens. Matter 9, 4145–4156 (1997).
Zanettini, S. et al. Magnetoconductance anisotropy of a polymer thin film at the onset of metallicity. Appl. Phys. Lett. 106, 063303 (2015).
Kang, K. et al. 2D coherent charge transport in highly ordered conducting polymers doped by solid state diffusion. Nat. Mater. 15, 896–902 (2016).
Ji, X. et al. Pauli paramagnetism of stable analogues of pernigraniline salt featuring ladder-type constitution. J. Am. Chem. Soc. 142, 641–648 (2020).
Moraes, F. et al. Doped poly(thiophene): electron spin resonance determination of the magnetic susceptibility. Synth. Met. 10, 169–179 (1985).
Tanaka, H., Hirate, M., Watanabe, S. & Kuroda, S.-I. Microscopic signature of metallic state in semicrystalline conjugated polymers doped with fluoroalkylsilane molecules. Adv. Mater. 26, 2376–2383 (2014).
Jin, E. et al. Two-dimensional sp2 carbon–conjugated covalent organic frameworks. Science 357, 673–676 (2017).
Aleshin, A. N., Kozub, V. I., Suh, D. S. & Park, Y. W. Saturation of dephasing and magnetoresistance features in heavily doped polyacetylene. Synth. Met. 135–136, 303–304 (2003).
Suh, D. S. et al. Linear high-field magnetoconductivity of doped polyacetylene up to 30 Tesla. Phys. Rev. B 65, 165210 (2002).
Wu, H.-Y. et al. Influence of molecular weight on the organic electrochemical transistor performance of ladder-type conjugated polymers. Adv. Mater. 34, 2106235 (2022).
Feng, K. et al. Cyano-functionalized n-type polymer with high electron mobility for high-performance organic electrochemical transistors. Adv. Mater. 34, 2201340 (2022).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian, Inc., 2016).
Mukherjee, A. K. & Menon, R. Magnetotransport in doped polyaniline. J. Phys. Condens. Matter 17, 1947–1960 (2005).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 71, 3–8 (2015).
Rivnay, J., Noriega, R., Kline, R. J., Salleo, A. & Toney, M. F. Quantitative analysis of lattice disorder and crystallite size in organic semiconductor thin films. Phys. Rev. B 84, 045203 (2011).
Noriega, R. et al. A general relationship between disorder, aggregation and charge transport in conjugated polymers. Nat. Mater. 12, 1038–1044 (2013).
Chen, Z. et al. Evolution of the electronic structure in open-shell donor-acceptor organic semiconductors. Nat. Commun. 12, 5889 (2021).
Elliott, R. J. Theory of the effect of spin-orbit coupling on magnetic resonance in some semiconductors. Phys. Rev. 96, 266–279 (1954).
Lee, P. A. & Ramakrishnan, T. V. Disordered electronic systems. Rev. Mod. Phys. 57, 287–337 (1985).
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (no. 2019YFA0705900) funded by MOST, the Basic and Applied Basic Research Major Program of Guangdong Province (no. 2019B030302007) and the National Natural Science Foundation of China (no. U21A6002).
Author information
Authors and Affiliations
Contributions
H.T. conceived the project and conducted materials synthesis and characterizations under the supervision of Y.C. and F.H. H.T., C.L. and Z.H. discussed and analysed data. Y.L. fabricated and measured the OECTs. Y.D. and Y.Z. conducted the SQUID and M–H measurements. A.S. and H.Z. assisted the synthesis and characterization of oligomers. D.Z. and Y.M. performed parts of the Seebeck coefficient measurements. H.T., F.H., H.G., X.G. and J.P. discussed the mechanism in PBFDO synthesis and its charge-transport mechanism. J.P. and Z.Y. helped verify the conductivity and Hall effect. H.T., C.L. and F.H. wrote the draft of the paper and all authors read and approved the paper.
Corresponding author
Ethics declarations
Competing interests
H.T. and F.H. have filed a PCT patent application (no. PCT/CN2021/124545). The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Simone Fabiano, Jianguo Mei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Investigations of reaction mechanisms.
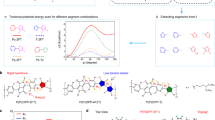
a, In situ monitoring of change in absorption for reactions in DMSO (with TMQ and protected by argon). (Note: the concentration of H-BFDO at the beginning was 5 mg ml−1 and the reaction mixture was left to stand without stirring to prevent influence on absorption collection.) b, The chemical structure of oligomers in different solvents obtained in the model reaction. c, The UV–vis absorption of different oligomers. Addition of 2,3,5,6-tetramethylbenzene-1,4-diol (TMQH, 10 mg ml−1 in DMF) in the undoped oligomers part (oligomers (THF), heating at 80 °C for 30 min after addition) would cause the increase of absorption around 900 nm (blue line), verifying the electron-transfer n-doping process from TMQH to the polymer. d, The corresponding electrochemical cyclic voltammetry curves of oligomers. The oxidation of oligomers (DMF) was not shown, owing to the very opaque signal, which is consistent with the oxidation of PBFDO (Fig. S42) and was attributed to the doping and conductive states of the material. e, Calculated distribution of LUMO profiles and energy levels of oligomers with different repeating units. Benefitting from the rigid conjugated backbones (dihedral angles between adjacent units are less than 6°), the LUMO could delocalize over more than six repeating units, which promoted electron transport and delocalization of negative (bi)polarons. Moreover, with increasing repeating units, the LUMO levels of oligomers decreased sharply, which was in accordance with the experimental results and would promote the electron-transfer doping from hydroquinone derivatives to the polymers.
Extended Data Fig. 2 The possible mechanisms of combined oxidative polymerization and reductive n-doping for the synthesis of PBFDO.
The reaction begins from TMQ-promoted lactone dimerization through a radical pathway, which is followed by oxidative dehydrogenation. Along with the oxidative polymerization proceeding, the formed polymer can be doped with the generated TMQH simultaneously. Doping also makes the doped polymer soluble in DMSO.
Extended Data Fig. 3 The microstructure analysis of PBFDO films.
a,b, Height image and current image of PBFDO films. c,d, GIWAXS characterization of PBFDO films deposited on Si substrates. e, Corresponding (100) lamellar packing analysis of PBFDO films. f, (010) π–π packing analysis of PBFDO films.
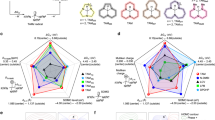
Extended Data Fig. 4 Investigations of transport mechanisms in PBFDO.
a,b, ESR spectroscopy of PBFDO at different temperatures. The ESR intensities of the PBFDO solid slightly increased with elevating temperature from 140 to 260 K (a), which was considered as a signature of open-shell diradical resonance54. Another phenomenon of line broadening of the ESR signal was observed above 298 K, implying the existence of conduction electrons, which is a typical signature in CPs25,42 (b). These conduction electrons are increasingly scattered by phonons with increasing temperature, resulting in a shortening of the spin-lattice relaxation time and, hence, a decrease in conductivity and broadening of the ESR signals above 298 K (ref. 55). c, The total electromagnetic-wave shielding percentage versus frequency. d, Electromagnetic-interference shielding efficiency (EMI SE) of PBFDO. SEr represents shielding efficiency from reflection and SEa represents that from absorption. The outstanding SE of PBFDO can be considered as another characteristic of metallic state. e,f, Temperature dependence of phase coherence time, τφ (e), and coherence length, Lφ (f), extracted from MC. Inelastic scattering would interfere with the coherence of delocalized charge wave, especially when the Lφ is comparable with the magnetic length, LB = (ħ/eB)1/2 ≈ 25.66 B−1/2 nm (ref. 56). g,h, The conductivity of PBFDO film under the applied magnetic field B = 5 T (g) (the values were corrected using the conductivity under B = −5 T to eliminate the deviation caused by the Hall effect; the error bar represents the standard deviation caused by the uncertainty of film thickness) and corresponding log–log plot of W versus temperature (h). The zero-field conductivity was also measured for the same sample, exhibiting the characteristic of critical region, thus eliminating the deviations between different samples.
Extended Data Fig. 5 The stability characterizations of PBFDO.
a, Stability of a PBFDO film under ambient environment (25 °C, 60% relative humidity). b, Stability of PBFDO films washed by common organic solvents through spin-coating (THF, chloroform (CF), chlorobenzene (CB), acetonitrile (MeCN) and water), showing changes in conductivity. Error bars indicate the standard deviations of six experimental replicates. c, Stability of PBFDO films dealt with acid or ammonia (thickness is around 3 μm). The film was immersed in the corresponding solution for 1 min and washed with water. Error bars indicate the standard deviations of six experimental replicates. The large error bar of PBFDO immersed in conc. H2SO4 may be caused by destroyed film morphology.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1–58, Supplementary Tables 1–6 and Supplementary References.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, H., Liang, Y., Liu, C. et al. A solution-processed n-type conducting polymer with ultrahigh conductivity. Nature 611, 271–277 (2022). https://doi.org/10.1038/s41586-022-05295-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05295-8
This article is cited by
-
Electrochemical benefits of conductive polymers as a cathode material in LFP battery technology
Journal of Solid State Electrochemistry (2024)
-
Heating up the binder
Nature Energy (2023)
-
Flexible organic field-effect transistors-based biosensors: progress and perspectives
Analytical and Bioanalytical Chemistry (2023)
-
Metal-Backboned Polymer: Conception, Design and Synthesis
Chinese Journal of Polymer Science (2023)
-
Nitrogen-doped graphite-like carbon derived from phthalonitrile resin with controllable negative magnetoresistance and negative permittivity
Advanced Composites and Hybrid Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.