Abstract
Accurate knowledge of the mineralogy is essential for understanding the lower mantle, which represents more than half of Earth’s volume. CaSiO3 perovskite is believed to be the third-most-abundant mineral throughout the lower mantle, following bridgmanite and ferropericlase1,2,3. Here we experimentally show that the calcium solubility in bridgmanite increases steeply at about 2,300 kelvin and above 40 gigapascals to a level sufficient for a complete dissolution of all CaSiO3 component in pyrolite into bridgmanite, resulting in the disappearance of CaSiO3 perovskite at depths greater than about 1,800 kilometres along the geotherm4,5. Hence we propose a change from a two-perovskite domain (TPD; bridgmanite plus CaSiO3 perovskite) at the shallower lower mantle to a single-perovskite domain (SPD; calcium-rich bridgmanite) at the deeper lower mantle. Iron seems to have a key role in increasing the calcium solubility in bridgmanite. The temperature-driven nature can cause large lateral variations in the depth of the TPD-to-SPD change in response to temperature variations (by more than 500 kilometres). Furthermore, the SPD should have been thicker in the past when the mantle was warmer. Our finding requires revision of the deep-mantle mineralogy models and will have an impact on our understanding of the composition, structure, dynamics and evolution of the region.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Datasets for this research are available online at https://doi.org/10.5281/zenodo.6979058.
References
Kesson, S., Gerald, J. F. & Shelley, J. Mineralogy and dynamics of a pyrolite lower mantle. Nature 393, 252–255 (1998).
Ricolleau, A. et al. Density profile of pyrolite under the lower mantle conditions. Geophys. Res. Lett. 36, L06302 (2009).
Irifune, T. et al. Iron partitioning and density changes of pyrolite in Earth’s lower mantle. Science 327, 193–195 (2010).
Brown, J. & Shankland, T. Thermodynamic parameters in the Earth as determined from seismic profiles. Geophys. J. Int. 66, 579–596 (1981).
Katsura, T., Yoneda, A., Yamazaki, D., Yoshino, T. & Ito, E. Adiabatic temperature profile in the mantle. Phys. Earth Planet. Interiors 183, 212–218 (2010).
Lee, K. K. et al. Equations of state of the high-pressure phases of a natural peridotite and implications for the Earth’s lower mantle. Earth Planet. Sci. Lett. 223, 381–393 (2004).
Ono, S., Ohishi, Y., Isshiki, M. & Watanuki, T. In situ X-ray observations of phase assemblages in peridotite and basalt compositions at lower mantle conditions: implications for density of subducted oceanic plate. J. Geophys. Res. Solid Earth 110, B02208 (2005).
Sinmyo, R. & Hirose, K. Iron partitioning in pyrolitic lower mantle. Phys. Chem. Miner. 40, 107–113 (2013).
Irifune, T. et al. High-pressure phase transformation in CaMgSi2O6 and implications for origin of ultra-deep diamond inclusions. Geophys. Res. Lett. 27, 3541–3544 (2000).
Vitos, L. et al. Phase transformations between garnet and perovskite phases in the Earth’s mantle: a theoretical study. Phys. Earth Planet. Interiors 156, 108–116 (2006).
Jung, D. Y. & Schmidt, M. W. Solid solution behaviour of CaSiO3 and MgSiO3 perovskites. Phys. Chem. Miner. 38, 311–319 (2011).
Muir, J. M., Thomson, A. R. & Zhang, F. The miscibility of calcium silicate perovskite and bridgmanite: a single perovskite solid solution in hot, iron-rich regions. Earth Planet. Sci. Lett. 566, 116973 (2021).
McDonough, W. F. & Sun, S.-S. The composition of the Earth. Chem. Geol. 120, 223–253 (1995).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Badro, J. et al. Electronic transitions in perovskite: possible nonconvecting layers in the lower mantle. Science 305, 383–386 (2004).
Ricolleau, A. et al. Phase relations and equation of state of a natural MORB: implications for the density profile of subducted oceanic crust in the Earth’s lower mantle. J. Geophys. Res. Solid Earth 115, B08202 (2010).
Murakami, M., Hirose, K., Kawamura, K., Sata, N. & Ohishi, Y. Post-perovskite phase transition in MgSiO3. Science 304, 855–858 (2004).
Lekic, V., Cottaar, S., Dziewonski, A. & Romanowicz, B. Cluster analysis of global lower mantle tomography: a new class of structure and implications for chemical heterogeneity. Earth Planet. Sci. Lett. 357, 68–77 (2012).
Shim, S.-H., Duffy, T. S. & Shen, G. The post-spinel transformation in Mg2SiO4 and its relation to the 660-km seismic discontinuity. Nature 411, 571–574 (2001).
Kurnosov, A., Marquardt, H., Frost, D. J., Ballaran, T. B. & Ziberna, L. Evidence for a Fe3+-rich pyrolitic lower mantle from (Al, Fe)-bearing bridgmanite elasticity data. Nature 543, 543–546 (2017).
Mashino, I., Murakami, M., Miyajima, N. & Petitgirard, S. Experimental evidence for silica-enriched Earth’s lower mantle with ferrous iron dominant bridgmanite. Proc. Natl Acad. Sci. USA 117, 27899–27905 (2020).
Dziewonski, A. M. & Anderson, D. L. Preliminary reference Earth model. Phys. Earth Planet. Interiors 25, 297–356 (1981).
Kasting, J. F. What caused the rise of atmospheric O2? Chem. Geol. 362, 13–25 (2013).
Korenaga, J. Initiation and evolution of plate tectonics on Earth: theories and observations. Annu. Rev. Earth Planet. Sci. 41, 117–151 (2013).
Korenaga, J. Urey ratio and the structure and evolution of Earth’s mantle. Rev. Geophys. 46, RG2007 (2008).
Davies, G. F. Effect of plate bending on the Urey ratio and the thermal evolution of the mantle. Earth Planet. Sci. Lett. 287, 513–518 (2009).
Andrault, D., Monteux, J., Le Bars, M. & Samuel, H. The deep Earth may not be cooling down. Earth Planetary Sci. Lett. 443, 195–203 (2016).
Lobanov, S. S. et al. Blocked radiative heat transport in the hot pyrolitic lower mantle. Earth Planet. Sci. Lett. 537, 116176 (2020).
Andrault, D. et al. Solidus and liquidus profiles of chondritic mantle: implication for melting of the Earth across its history. Earth Planet. Sci. Lett. 304, 251–259 (2011).
Kim, T. et al. Low melting temperature of anhydrous mantle materials at the core–mantle boundary. Geophys. Res. Lett. 47, e2020GL089345 (2020).
Tangeman, J. A. et al. Vitreous forsterite (Mg2SiO4): synthesis, structure, and thermochemistry. Geophys. Res. Lett. 28, 2517–2520 (2001).
Herzberg, C., Condie, K. & Korenaga, J. Thermal history of the Earth and its petrological expression. Earth Planet. Sci. Lett. 292, 79–88 (2010).
Johnson, T. E., Brown, M., Kaus, B. J. & VanTongeren, J. A. Delamination and recycling of Archaean crust caused by gravitational instabilities. Nat. Geosci. 7, 47–52 (2014).
Ko, B. et al. Mineralogy and density of Archean volcanic crust in the mantle transition zone. Phys. Earth Planet. Interiors 305, 106490 (2020).
Rivers, M. et al. The COMPRES/GSECARS gas-loading system for diamond anvil cells at the Advanced Photon Source. High Press. Res. 28, 273–292 (2008).
Prakapenka, V. et al. Advanced flat top laser heating system for high pressure research at GSECARS: application to the melting behavior of germanium. High Press. Res. 28, 225–235 (2008).
Meng, Y., Hrubiak, R., Rod, E., Boehler, R. & Shen, G. New developments in laser-heated diamond anvil cell with in situ synchrotron X-ray diffraction at High Pressure Collaborative Access Team. Rev. Sci. Instrum. 86, 072201 (2015).
Prescher, C. & Prakapenka, V. B. Dioptas: a program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 35, 223–230 (2015).
Shim, S.-H. PeakPo—a python software for X-ray diffraction analysis at high pressure and high temperature. Zenodo https://doi.org/10.5281/zenodo.842949 (2017).
Holland, T. & Redfern, S. UNITCELL: a nonlinear least-squares program for cell-parameter refinement and implementing regression and deletion diagnostics. J. Appl. Crystallogr. 30, 84–84 (1997).
Toby, B. H. & Von Dreele, R. B. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 46, 544–549 (2013).
Dorogokupets, P. & Dewaele, A. Equations of state of MgO, Au, Pt, NaCl-B1, and NaCl-B2: internally consistent high-temperature pressure scales. High Press. Res. 27, 431–446 (2007).
Shim, S.-H. Pytheos—a Python tool set for equations of state. Zenodo https://doi.org/10.5281/zenodo.802392 (2017).
Sinmyo, R. & Hirose, K. The Soret diffusion in laser-heated diamond-anvil cell. Phys. Earth Planet. Interiors 180, 172–178 (2010).
Stamenkovic, V., Breuer, D. & Spohn, T. Thermal and transport properties of mantle rock at high pressure: applications to super-earths. Icarus 216, 572–596 (2011).
Dorfman, S. M., Meng, Y., Prakapenka, V. B. & Duffy, T. S. Effects of Fe-enrichment on the equation of state and stability of (Mg,Fe)SiO3 perovskite. Earth Planet. Sci. Lett. 361, 249–257 (2013).
Newville, M. et al. LMFIT: Non-linear least-square minimization and curve-fitting for Python. Astrophysics Source Code Library ascl:1606.014 (2016).
Irifune, T., Ringwood, A. & Hibberson, W. Subduction of continental crust and terrigenous and pelagic sediments: an experimental study. Earth Planet. Sci. Lett. 126, 351–368 (1994).
Nishiyama, N. & Yagi, T. Phase relation and mineral chemistry in pyrolite to 2200 °C under the lower mantle pressures and implications for dynamics of mantle plumes. J. Geophys. Res. Solid Earth 108, 2255 (2003).
Nishiyama, N., Irifune, T., Inoue, T., Ando, J.-I. & Funakoshi, K.-I. Precise determination of phase relations in pyrolite across the 660 km seismic discontinuity by in situ X-ray diffraction and quench experiments. Phys. Earth Planet. Interiors 143, 185–199 (2004).
Ono, S., Ohishi, Y. & Mibe, K. Phase transition of Ca-perovskite and stability of Al-bearing Mg-perovskite in the lower mantle. Am. Mineral. 89, 1480–1485 (2004).
Murakami, M., Hirose, K., Sata, N. & Ohishi, Y. Post-perovskite phase transition and mineral chemistry in the pyrolitic lowermost mantle. Geophys. Res. Lett. 32, L03304 (2005).
Ono, S. & Oganov, A. R. In situ observations of phase transition between perovskite and CaIrO3-type phase in MgSiO3 and pyrolitic mantle composition. Earth Planet. Sci. Lett. 236, 914–932 (2005).
Ohta, K., Hirose, K., Lay, T., Sata, N. & Ohishi, Y. Phase transitions in pyrolite and MORB at lowermost mantle conditions: implications for a MORB-rich pile above the core–mantle boundary. Earth Planet. Sci. Lett. 267, 107–117 (2008).
Kubo, A., Ito, E., Katsura, T., Fujino, K. & Funakoshi, K.-I. In situ X-ray diffraction of pyrolite to 40 GPa using Kawai-type apparatus with sintered diamond anvils: possibility for the existence of iron-rich metallic particles in the lower mantle. High Press. Res. 28, 351–362 (2008).
Sanehira, T. et al. Density profiles of pyrolite and MORB compositions across the 660 km seismic discontinuity. High Press. Res. 28, 335–349 (2008).
Ohta, K. et al. Electrical conductivities of pyrolitic mantle and MORB materials up to the lowermost mantle conditions. Earth Planet. Sci. Lett. 289, 497–502 (2010).
Auzende, A.-L. et al. Synthesis of amorphous MgO-rich peridotitic starting material for laser-heated diamond anvil cell experiments—application to iron partitioning in the mantle. High Press. Res. 31, 199–213 (2011).
Sinmyo, R., Hirose, K., Muto, S., Ohishi, Y. & Yasuhara, A. The valence state and partitioning of iron in the Earth’s lowermost mantle. J. Geophys. Res. Solid Earth 116, B07205 (2011).
Nabiei, F. et al Investigating magma ocean solidification on Earth through laser‐heated diamond anvil cell experiments. Geophys. Res. Lett. 48, e2021GL092446 (2021).
Stacey, F. D. A thermal model of the Earth. Phys. Earth Planet. Interiors 15, 341–348 (1977).
Asahara, Y. et al. Formation of metastable cubic-perovskite in high-pressure phase transformation of Ca(Mg,Fe,Al)Si2O6. Am. Mineral. 90, 457–462 (2005).
Sano, A. et al. In situ X-ray diffraction study of the effect of water on the garnet–perovskite transformation in MORB and implications for the penetration of oceanic crust into the lower mantle. Phys. Earth Planet. Interiors 159, 118–126 (2006).
Chen, H. et al. Crystal structure of CaSiO3 perovskite at 28–62 GPa and 300 K under quasi-hydrostatic stress conditions. Am. Mineral. 103, 462–468 (2018).
Shim, S.-H., Jeanloz, R. & Duffy, T. S. Tetragonal structure of CaSiO3 perovskite above 20 GPa. Geophys. Res. Lett. 29, 19-1–19-4 (2002).
Dorfman, S. M., Shieh, S. R., Meng, Y., Prakapenka, V. B. & Duffy, T. S. Synthesis and equation of state of perovskites in the (Mg,Fe)3Al2Si3O12 system to 177 GPa. Earth Planet. Sci. Lett. 357, 194–202 (2012).
O’keeffe, M., Hyde, B. & Bovin, J.-O. Contribution to the crystal chemistry of orthorhombic perovskites: MgSiO3 and NaMgF3. Phys. Chem. Miner. 4, 299–305 (1979).
Walter, M. et al. Phase relations and equation-of-state of aluminous Mg-silicate perovskite and implications for Earth’s lower mantle. Earth Planet. Sci. Lett. 222, 501–516 (2004).
Catalli, K. et al. Spin state of ferric iron in MgSiO3 perovskite and its effect on elastic properties. Earth Planet. Sci. Lett. 289, 68–75 (2010).
Knittle, E. & Jeanloz, R. Synthesis and equation of state of (Mg,Fe)SiO3 perovskite to over 100 gigapascals. Science 235, 668–670 (1987).
Andrault, D., Bolfan-Casanova, N. & Guignot, N. Equation of state of lower mantle (Al,Fe)-MgSiO3 perovskite. Earth Planet. Sci. Lett. 193, 501–508 (2001).
Nomura, R. et al. Spin crossover and iron-rich silicate melt in the Earth’s deep mantle. Nature 473, 199–202 (2011).
Sturhahn, W. CONUSS and PHOENIX: evaluation of nuclear resonant scattering data. Hyperfine Interact. 125, 149–172 (2000).
Hsu, H., Blaha, P., Cococcioni, M. & Wentzcovitch, R. M. Spin-state crossover and hyperfine interactions of ferric iron in MgSiO3 perovskite. Phys. Rev. Lett. 106, 118501 (2011).
Sinmyo, R., McCammon, C. & Dubrovinsky, L. The spin state of Fe3+ in lower mantle bridgmanite. Am. Mineral. J. Earth Planet. Mater. 102, 1263–1269 (2017).
Liu, J. et al. Valence and spin states of iron are invisible in Earth’s lower mantle. Nat. Commun. 9, 1284 (2018).
Fujino, K. et al. Spin transition of ferric iron in Al-bearing Mg–perovskite up to 200 GPa and its implication for the lower mantle. Earth Planet. Sci. Lett. 317, 407–412 (2012).
Acknowledgements
K. Mossman and M. R. Gutierrez assisted with the FIB and STEM measurements at Arizona State University; and Y.-J. Chang assisted with the FIB and STEM measurements at University of Arizona. This work was supported by National Science Foundation (EAR-1725094). This research used resources of the Advanced Photon Source (APS), a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract number DE-AC02-06CH11357. We acknowledge the support of GeoSoilEnviroCARS (Sector 13), which is supported by the National Science Foundation (NSF) - Earth Sciences (EAR-1634415), and the Department of Energy, Geosciences (DE-FG02-94ER14466). Use of the COMPRES-GSECARS gas loading system was also supported by COMPRES under NSF Cooperative Agreement EAR -1606856. High-Pressure Collaborative Access Team (Sector 16) is supported by DOE-NNSA Grant DE-NA0001974 and DOE-BES Grant DE-FG02-99ER45775.
Author information
Authors and Affiliations
Contributions
B.K. conceptualized the project, designed and performed the experiments, analysed data and wrote the manuscript. S.-H.S. conceptualized and supervised the project, acquired funding, designed experiments and wrote the manuscript. E.G., V.P., Y.M. and D.Z. provided resources for the in situ XRD measurements and supervised the experiments. E.E.A. and W.B. provided resources for the SMS measurements and supervised the experiments. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Previous reports of existence (small symbols) or absence (large symbols) of CaSiO3 perovskite from experiments on pyrolitic and peridotitic compositions.
The data are collected from refs. 2,3,6,7,8,28,48,49,50,51,52,53,54,55,56,57,58,59. Mantle geotherms are shown as a grey area with the lower bound from ref. 4. and the upper bound from ref. 5. Diamonds: bridgmanite + ferropericlase + CaSiO3 perovskite. Circles: bridgmanite + ferropericlase or bridgmanite only. Squares: bridgmanite (+ post-perovskite) + ferropericlase + CaSiO3 perovskite. Pentagons: bridgmanite (+ post-perovskite) + ferropericlase. The open symbols are from this study.
Extended Data Fig. 2 In situ X-ray diffraction patterns measured at high P−T conditions for the (a) komatiitic composition and (b) Ca-pyrolite.
a,b, Miller indices of bridgmanite, CaSiO3 perovskite, and Rh2O3(II)-type Al2O3 are provided as the black, blue, and magenta labels. The diffraction peak positions of CaPv are shown as the blue bars.
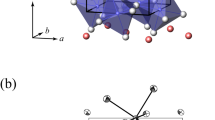
Extended Data Fig. 3 Effects of Ca dissolution on crystal structure of bridgmanite.
a,b, The molar volumes of bridgmanite (Brg) at high pressures and 300 K. The red and blue symbols represent Ca-rich Brg and Brg, respectively. The solid curves are fits of the solid symbol data points to the Vinet equation with fixed K0′ = 4. The data shown as open symbols were not used for fitting. The calculated molar volume of the Brg + CaSiO3 perovskite (CaPv) assemblage is plotted as the dashed black curve for comparison with uncertainties (the grey shade). a, The blue dashed curve is from Mg,Fe,Al-bearing Brg in KLB-1 peridotite2. b, The inset is a magnified view for the purple triangles. c,d, The octahedral tilting of Brg at high pressures and 300 K. The octahedral tilting angle for MgSiO3 endmember Brg is shown as the black dashed line. d, The red circles (K62 and K100) and diamonds (K59B and K73B) denote Ca-rich Brg. The blue circles (K33) and diamonds (K59B and K73B) denote Brg. b,d, The purple triangles represent the data points of Ca-rich Brg observed together with CaPv in XRD patterns (K100-2; Methods). The error bars are estimated 1σ uncertainties.
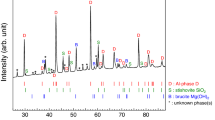
Extended Data Fig. 4 X-ray diffraction patterns and chemical analysis of komatiitic composition synthesized at 62 GPa and 2,350 K (K62) and 48 GPa and 2,000 K (K48).
a,b, Miller indices of Ca-rich bridgmanite (Brg), CaSiO3 perovskite (CaPv), stishovite (St), and corundum (Crn) are provided as the black, blue, red, and magenta labels, respectively. The expected diffraction peak positions of CaPv are shown as the blue bars. c,d, A high-angle angular dark-field image (left) and chemical maps (right) of the recovered samples. A small grain of CaPv is rarely observed in c, as the majority of Ca exists in Ca-Brg because of an increase in Ca solubility in Brg with temperature. However, at lower temperature CaPv is frequently observed because of low Ca solubility in Brg at the conditions.
Extended Data Fig. 5 X-ray diffraction patterns of the Ca30Fe13 composition at high pressures and 300 K.
Pressures were measure at 300 K after heating. The Miller indices of bridgmanite and CaSiO3 perovskite are provided as the black and blue labels, respectively. The expected diffraction peak positions of CaSiO3 perovskite (tetragonal, space group: I4/mcm) is shown as the blue bars.
Extended Data Fig. 6 Synchrotron Mössbauer spectra of bridgmanite (Brg) and Ca-rich Brg at 59 GPa and at 58 GPa after laser heating.
The synthesis temperatures were 2,150 K and 2,400 K for Brg and Ca-rich Brg, respectively. The circles are measured spectral data points and the curves are spectral fitting results.
Extended Data Fig. 7 Differences in the seismic properties between the single-perovskite phase (Ca-rich bridgmanite) case and the two perovskite phases (bridgmanite + CaSiO3 perovskite) case.
The differences of Ca-rich bridgmanite from bridgmanite + CaSiO3 perovskite are shown for bulk sound speed (Φ), bulk modulus (KT), and density (ρ) for the komatiitic composition at 300 K. The grey shades show the estimated 1σ uncertainties.
Extended Data Fig. 8 The depth of the transition zone from a two-perovskite domain (TPD; bridgmanite + CaSiO3 perovskite) at shallower depths to a single-perovskite domain (SPD; Ca-rich bridgmanite) at greater depths over time in the lower mantle.
a, The depth of the TPD-to-SPD transition zone (TSTZ) was calculated for the mantle geotherms from ref. 4 (green) and ref. 5 (orange) for Urey ratios (Ur) of 0.23, 0.38 (ref. 25) and 0.8 (ref. 26). The thickness of the TSTZ was not considered for the calculation. b, A 3-D plot of the TSTZ depths for Ur = 0.38. The green and orange planes intersect the grey plane which represents the TSTZ at 2,300 K.
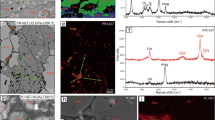
Extended Data Fig. 9 High-angle annular-dark-field images of the samples of Ca-pyrolite (a) and komatiitic composition (b).
a,b, The yellow boxes indicate the areas where chemical compositions were measured, which are presented in Fig. 2b, d. a, Chemical compositions were analysed at the bottom left of the sample where the thickness was smaller. The presented area of the sample shows texture consistent with heating centre, that is, well crystallized bridgmanite and ferropericlase. b, Chemical compositions were analysed at the top area of the sample. The Ca-rich bridgmanite matrix is nearly indistinguishable from the unheated glass since the Ca-rich bridgmanite matrix takes roughly 95 vol% of the sample. However, the well crystallized grains of other phases (Al2O3 and SiO2) show that the area is well heated.
Supplementary information
Supplementary Tables 1–5
The excel file contains Supplementary Tables 1–5. The individual tables are displayed in separate spreadsheets. The tables provide detailed experimental data to support the main text.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ko, B., Greenberg, E., Prakapenka, V. et al. Calcium dissolution in bridgmanite in the Earth’s deep mantle. Nature 611, 88–92 (2022). https://doi.org/10.1038/s41586-022-05237-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05237-4
This article is cited by
-
Full-waveform tomography reveals iron spin crossover in Earth’s lower mantle
Nature Communications (2024)
-
Variation in bridgmanite grain size accounts for the mid-mantle viscosity jump
Nature (2023)
-
Solubility of water in bridgmanite
Acta Geochimica (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.