Abstract
The phytohormone auxin triggers transcriptional reprogramming through a well-characterized perception machinery in the nucleus. By contrast, mechanisms that underlie fast effects of auxin, such as the regulation of ion fluxes, rapid phosphorylation of proteins or auxin feedback on its transport, remain unclear1,2,3. Whether auxin-binding protein 1 (ABP1) is an auxin receptor has been a source of debate for decades1,4. Here we show that a fraction of Arabidopsis thaliana ABP1 is secreted and binds auxin specifically at an acidic pH that is typical of the apoplast. ABP1 and its plasma-membrane-localized partner, transmembrane kinase 1 (TMK1), are required for the auxin-induced ultrafast global phospho-response and for downstream processes that include the activation of H+-ATPase and accelerated cytoplasmic streaming. abp1 and tmk mutants cannot establish auxin-transporting channels and show defective auxin-induced vasculature formation and regeneration. An ABP1(M2X) variant that lacks the capacity to bind auxin is unable to complement these defects in abp1 mutants. These data indicate that ABP1 is the auxin receptor for TMK1-based cell-surface signalling, which mediates the global phospho-response and auxin canalization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within this paper and its Supplementary Information files (Excel files with source data for all graphs, uncropped blots and gel images). Proteomics data are deposited at Proteome Exchange (PXD031063). Sequence data from this article can be found in the Arabidopsis Genome Initiative databases under the following accession numbers: AT4G02980 (ABP1), AT1G66150 (TMK1), AT1G24650 (TMK2), AT2G01820 (TMK3), AT3G23750 (TMK4), AT3G14470 (LRR4), AT2G18960 (AHA1), AT4G30190 (AHA2), AT5G20490 (myosin XIK), AT1G62390 (MadB2/PHOX2), AT2G25290 (MadB1/PHOX1) and AT5G20360 (MadB/PHOX3). Correspondence and requests for materials should be addressed to J.F. Source data are provided with this paper.
References
Friml, J. Fourteen stations of auxin. Cold Spring Harb. Perspect. Biol. 14, a039859 (2021).
Lavy, M. & Estelle, M. Mechanisms of auxin signaling. Development 143, 3226–3229 (2016).
Morffy, N. & Strader, L. C. Structural aspects of auxin signaling. Cold Spring Harb. Perspect. Biol. 14, a039883 (2021).
Napier, R. The story of auxin-binding protein 1 (ABP1). Cold Spring Harb. Perspect. Biol. 13, a039909 (2021).
Fendrych, M. et al. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat. Plants 4, 453–459 (2018).
Dindas, J. et al. AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nat. Commun. 9, 1174 (2018).
Gallei, M., Luschnig, C. & Friml, J. Auxin signalling in growth: Schrödinger’s cat out of the bag. Curr. Opin. Plant Biol. 53, 43–49 (2020).
Li, L., Gallei, M. & Friml, J. Bending to auxin: fast acid growth for tropisms. Trends Plant Sci. 27, 440–449 (2022).
Kuhn, A. et al. Direct ETTIN–auxin interaction controls chromatin states in gynoecium development. eLife 9, e51787 (2020).
Cao, M. et al. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 568, 240–243 (2019).
Dubey, S. M., Serre, N. B. C., Oulehlová, D., Vittal, P. & Fendrych, M. No time for transcription-rapid auxin responses in plants. Cold Spring Harb. Perspect. Biol. 13, a039891 (2021).
Adamowski, M. & Friml, J. PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27, 20–32 (2015).
Narasimhan, M. et al. Systematic analysis of specific and nonspecific auxin effects on endocytosis and trafficking. Plant Physiol. 186, 1122–1142 (2021).
Robert, S. et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143, 111–121 (2010).
Sachs, T. The induction of transport channels by auxin. Planta 127, 201–206 (1975).
Han, H. et al. Rapid auxin-mediated phosphorylation of myosin regulates trafficking and polarity in Arabidopsis. Preprint at bioRxiv https://doi.org/10.1101/2021.04.13.439603 (2021).
Li, L. et al. Cell surface and intracellular auxin signalling for H + fluxes in root growth. Nature 599, 273–277 (2021).
Lin, W. et al. TMK-based cell-surface auxin signaling activates cell wall acidification. Nature 599, 278–282 (2021).
McLaughlin, H. M., Ang, A. C. H. & Østergaard, L. Noncanonical auxin signaling. Cold Spring Harb. Perspect. Biol. 13, a039917 (2021).
Hertel, R., Thomson, K. S. & Russo, V. E. In-vitro auxin binding to particulate cell fractions from corn coleoptiles. Planta 107, 325–340 (1972).
Xu, T. et al. Cell surface ABP1–TMK auxin-sensing complex activates ROP GTPase signaling. Science 343, 1025–1028 (2014).
Gao, Y. et al. Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc. Natl Acad. Sci. USA 112, 2275–2280 (2015).
Grones, P. et al. Auxin-binding pocket of ABP1 is crucial for its gain-of-function cellular and developmental roles. J. Exp. Bot. 66, 5055–5065 (2015).
Michalko, J., Dravecká, M., Bollenbach, T. & Friml, J. Embryo-lethal phenotypes in early abp1 mutants are due to disruption of the neighboring BSM gene. F1000Res. 4, 1104 (2015).
Dai, X. et al. Embryonic lethality of Arabidopsis abp1-1 is caused by deletion of the adjacent BSM gene. Nature Plants 1, 15183 (2015).
Smakowska-Luzan, E. et al. An extracellular network of Arabidopsis leucine-rich repeat receptor kinases. Nature 553, 342–346 (2018).
Woo, E. J. et al. Crystal structure of auxin-binding protein 1 in complex with auxin. EMBO J. 21, 2877–2885 (2002).
Tian, H., Klambt, D. & Jones, A. M. Auxin-binding protein 1 does not bind auxin within the endoplasmic reticulum despite this being the predominant subcellular location for this hormone receptor. J. Biol. Chem. 270, 26962–26969 (1995).
Gelová, Z. et al. Developmental roles of auxin binding protein 1 in Arabidopsis thaliana. Plant Sci. 303, 110750 (2021).
Dahlke, R. I. et al. Protoplast swelling and hypocotyl growth depend on different auxin signaling pathways. Plant Physiol. 175, 982–994 (2017).
Jayakannan, M., Bose, J., Babourina, O., Rengel, Z. & Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 64, 2255–2268 (2013).
Paponov, I. A. et al. Auxin-induced plasma membrane depolarization is regulated by auxin transport and not by AUXIN BINDING PROTEIN1. Front. Plant Sci. 9, 1953 (2019).
Serre, N. B. C. et al. AFB1 controls rapid auxin signalling through membrane depolarization in Arabidopsis thaliana root. Nat. Plants 7, 1229–1238 (2021).
Tominaga, M. & Ito, K. The molecular mechanism and physiological role of cytoplasmic streaming. Curr. Opin. Plant Biol. 27, 104–110 (2015).
Sauer, M. et al. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 20, 2902–2911 (2006).
Mazur, E., Benková, E. & Friml, J. Vascular cambium regeneration and vessel formation in wounded inflorescence stems of Arabidopsis. Sci. Rep. 6, 33754 (2016).
Mazur, E. et al. Clathrin-mediated trafficking and PIN trafficking are required for auxin canalization and vascular tissue formation in Arabidopsis. Plant Sci. 293, 110414 (2020).
Mazur, E., Kulik, I., Hajný, J. & Friml, J. Auxin canalization and vascular tissue formation by TIR1/AFB-mediated auxin signaling in Arabidopsis. New Phytol. 226, 1375–1383 (2020).
Dai, N., Wang, W., Patterson, S. E. & Bleecker, A. B. The TMK subfamily of receptor-like kinases in Arabidopsis display an essential role in growth and a reduced sensitivity to auxin. PLoS One 8, e60990 (2013).
Hajný, J., Tan, S. & Friml, J. Auxin canalization: from speculative models toward molecular players. Curr. Opin. Plant Biol. 65, 102174 (2022).
Wabnik, K. et al. Emergence of tissue polarization from synergy of intracellular and extracellular auxin signaling. Mol. Syst. Biol. 6, 447 (2010).
Hajný, J. et al. Receptor kinase module targets PIN-dependent auxin transport during canalization. Science 370, 550–557 (2020).
Yin, K., Han, X., Xu, Z. & Xue, H. Arabidopsis GLP4 is localized to the Golgi and binds auxin in vitro. Acta Biochim. Biophys. Sin. 41, 478–487 (2009).
Friml, J. et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426, 147–153 (2003).
Benková, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003).
Klode, M., Dahlke, R. I., Sauter, M. & Steffens, B. Expression and subcellular localization of Arabidopsis thaliana auxin-binding protein 1 (ABP1). J. Plant Growth Regul. 30, 416–424 (2011).
Özkan, E. et al. An extracellular interactome of immunoglobulin and LRR proteins reveals receptor–ligand networks. Cell 154, 228–239 (2013).
Wasilko, D. J. et al. The titerless infected-cells preservation and scale-up (TIPS) method for large-scale production of NO-sensitive human soluble guanylate cyclase (sGC) from insect cells infected with recombinant baculovirus. Protein Expr. Purif. 65, 122–132 (2009).
Tan, S. et al. Salicylic acid targets protein phosphatase 2A to attenuate growth in plants. Curr. Biol. 30, 381–395.e8 (2020).
Jerabek-Willemsen, M., Wienken, C. J., Braun, D., Baaske, P. & Duhr, S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev. Technol. 9, 342–353 (2011).
Wiśniewski, J. R., Zougman, A., Nagaraj, N. & Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 (2009).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Vizcaíno, J. A. et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456 (2016).
Okumura, M. & Kinoshita, T. Measurement of ATP hydrolytic activity of plasma membrane H+-ATPase from Arabidopsis thaliana leaves. Bio-protocol 6, e2044 (2016).
Živanovic, B., Köhler, K., Galland, P. & Weisenseel, M. Membrane potential and endogenous ion current of Phycomyces sporangiophores. Electro. Magnetobiol. 20, 343–362 (2009).
Young, G. et al. Quantitative mass imaging of single biological macromolecules. Science 360, 423–427 (2018).
Kelly, S. M., Jess, T. J. & Price, N. C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 1751, 119–139 (2005).
Anthis, N. J. & Clore, G. M. Sequence-specific determination of protein and peptide concentrations by absorbance at 205 nm. Protein Sci. 22, 851–858 (2013).
Acknowledgements
We acknowledge K. Kubiasová for excellent technical assistance, J. Neuhold, A. Lehner and A. Sedivy for technical assistance with protein production and purification at Vienna Biocenter Core Facilities; Creoptix for performing GCI; and the Bioimaging, Electron Microscopy and Life Science Facilities at ISTA, the Plant Sciences Core Facility of CEITEC Masaryk University, the Core Facility CELLIM (MEYS CR, LM2018129 Czech-BioImaging) and J. Sprakel for their assistance. J.F. is grateful to R. Napier for many insightful suggestions and support. We thank all past and present members of the Friml group for their support and for other contributions to this effort to clarify the controversial role of ABP1 over the past seven years. The project received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 742985 to J.F. and 833867 to D.W.); the Austrian Science Fund (FWF; P29988 to J.F.); the Netherlands Organization for Scientific Research (NWO; VICI grant 865.14.001 to D.W. and VENI grant VI.Veni.212.003 to A.K.); the Ministry of Education, Science and Technological Development of the Republic of Serbia (contract no. 451-03-68/2022-14/200053 to B.D.Ž.); and the MEXT/JSPS KAKENHI to K.T. (20K06685) and T.K. (20H05687 and 20H05910).
Author information
Authors and Affiliations
Contributions
J.F. conceived and coordinated the experiments and wrote the paper with input from all authors. J.F., D.W. and T.K. interpreted the results. The following individuals conducted the experiments, analysed the data and contributed to their design and interpretation: A.J., P.G. and W.A.K. (TEM); A.K., D.W., L.F. and M. Roosjen (rapid phosphoproteomics); A.M. and L.R. (western blotting, cloning and analysis of kinase activity); A.T., J.F., M.G., M.H., M.N., M. Randuch and S.T. (phenotype analysis); B.D.Ž. (measurements of membrane potential); C.G. (mass photometry); H.R. (cloning, localization and phenotype analysis); E.M. and N.R. (regeneration and canalization analysis, GUS staining); I.V. (DARTS and MST); K.T. and T.K. (plasma-membrane ATPase activity); M.Z. (cytoplasmic streaming, circular dichroism analysis and GUS staining); and Z.G. (MST, western blotting, cloning and expression analysis).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Kenneth Birnbaum, Angus Murphy, Justin Walley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Analysis of auxin binding to Arabidopsis thaliana TMK1 and ABP1.
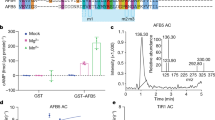
a, DARTS assay on TMK1::TMK1-GFP plants. Protein extracts were incubated with IAA (in blue) or benzoic acid (in grey). Afterwards, different pronase quantities were added. Blots and quantifications (normalized to actin levels) show comparable pronase-induced degradation in the presence of both IAA and benzoic acid, suggesting no specific IAA binding to TMK1. Representative of 3 independent experiments with similar outcome. b, MST analysis of auxin binding to TMK1. Normalized binding curves of IAA, NAA and benzoic acid in the presence of 150 nM TMK1 heterologously expressed and purified extracellular domain (ECD). Plots are mean ± SD from 10 measurements (3 or 4 independent experiments) but no curve fitting to determine binding kinetics was possible. c, GCI-assisted analysis of binding properties of TMK1 ECD to IAA or benzoic acid as a control ligand, using the Creoptix® WAVEsystem. Heterologously expressed and purified TMK1 ECD was immobilized to the surface at the indicated levels, following which the response to different concentrations of IAA or benzoic acid in running buffer at different pH (resp. 5.5 and 7.6) was monitored for analysis of binding kinetics. No binding of IAA to TMK1 ECD was detected. d, DARTS assay on heterologously expressed ABP1. Purified protein was mixed with different quantities of pronase enzyme mixture. Blots and quantifications show that pronase-induced proteolysis of tagged ABP1 occurred less in the presence of 10 μM IAA, which was consistent for multiple pronase dilutions, indicating IAA association with ABP1. This was verified both by the anti-HIS-HRP antibody to ensure specificity of the visualized band. The intensity profiles are plotted in the graph below the blots. Representative of 3 independent experiments with similar outcome shown. e, Control no-pronase samples for DARTS results represented in Fig. 1a. Protein extracts from 35S::ABP1-GFP expressing plants were incubated with benzoic acid (in grey) or IAA (in blue). Blot intensities were quantified and normalized to the average actin intensity for the no-pronase samples. In these no-pronase control samples, we verified that the presence of the respective potential ligand at their concentration did not affect target protein stability as such. Representative of 3 independent experiments with similar outcome. f, Overview table and graphs of all GCI-based binding analyses for ABP1. The potential ligands IAA and benzoic acid were evaluated in serial dilution ranging from 91.5 nM to 200 μM. IAA binding kinetics was detectable, giving a Kd estimate of 13.7 μM at pH 5.5 and 1943 μM at pH 7.6. g, MST analysis of IAA binding to ABP1 at varying pH. Normalized binding curve of IAA in presence of 75 nM ABP1 at pH 5.5 (blue), 100 nM ABP1 at pH 7.0 (grey) or 100 nM ABP1 pH 7.5 (black). The IAA concentration varied from 61 nM to 2 mM. Plots are mean ± SD from 10 measurements; 2 (pH 7.5) or 4 (pH 5.5, pH 7.0) independent experiments. The estimated Kd values confirm efficient IAA binding at the apoplastic pH of 5.5, whereas the estimated Kd values with large SD indicate no binding at the pH 7.0 and pH 7.5. h, MST analysis of ligand binding to ABP1 at pH 7.0. Normalized binding curve of IAA (blue; identical to grey in S1g) and L-Trp (black) to 100 nM ABP1. The ligand concentration varied from 61 nM to 2 mM. Plots are mean ± SD from 10 measurements; 4 independent experiments. The estimated Kd values with large SD indicate no binding of these ligands at pH 7.0. i, MST analysis of ligand binding to ABP1 at pH 7.5. Normalized binding curve of IAA (blue, same as black in Extended Data Fig. 1g) and control ligand benzoic acid (grey) to 75 nM ABP1. The ligand concentration varied from 61 nM to 2 mM. Plots are mean ± SD from 10 measurements; 2 independent experiments. The estimated Kd values and SDs are much higher than those obtained for pH 5.5 and indicate no binding of these ligands at pH 7.5.
Extended Data Fig. 2 TEM analysis of apoplastic ABP1 localization.
a, Example TEM images of RPS5A::ABP1-GFP root cells after mock and IAA (1 µM) incubation. Lower ‘Zoom’ panels are higher magnification images of apoplastic areas from RPS5A::ABP1-GFP (IAA) cells showing apoplastic anti-GFP signal. Arrows note gold particles. Scale bars; upper, 1 µm; lower, 500 nm. b, Quantification of the densities of apoplastic localized anti-GFP gold particles in root cells of WT and RPS5A::ABP1-GFP plants subjected to either mock or 1 µM IAA incubation, as detected by TEM. Plots are mean ± SEM. n: 3 experimental repeats. WT Mock, 37 images; WT IAA, 43 images; ABP1 mock, 45 images; ABP1 IAA, 47 images. Kruskal–Wallis analysis (χ2 = 99.59, df = 3, P = 1.90E-21) followed by uncorrected unpaired two-sided Wilcoxon rank-sum tests with continuity correction. WT mock versus WT IAA: P = 0.3659, WT mock versus ABP1 mock: P = 1.16E-11, WT IAA versus ABP1 IAA: P = 7.10E-13, ABP1 mock versus ABP1 IAA: P = 0.3118. ****P ≤ 0.0001. c, Example TEM image of an RPS5A::ABP1-GFP root cell showing anti-GFP gold labelling of the ER (arrows). Scale bar, 200 nm. d, Example TEM images of WT and ABP1::GFP-ABP1 shoot apical meristem cells labelled with anti-GFP immunogold particles (arrows). Mock or 1 µM IAA incubation. Scale bar, 200 nm.
Extended Data Fig. 3 Global, ultrafast auxin phospho-response and rapid cellular effects.
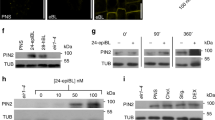
a, Ultrafast phosphoproteomics in mock-treated (2 min) Arabidopsis roots. Distributions of log2 fold changes for P-peptides with significantly differential phosphorylation (FDR < 0.05) in tmk1-1 and abp1-TD1 as compared to WT. Both mutants exhibit global hypo-phosphorylation. b, Mock treatment (2 min) of Arabidopsis roots. The volcano plot depicts log2 fold changes (x-axis; tmk1-1 versus WT) and statistical significance (y axis). Highlighted in red is a subset of significantly regulated P-peptides (FDR < 0.05) which are also significantly regulated in abp1-TD1/WT. This shows global hypo-phosphorylation in tmk1-1 and extensive functional overlap between the two mutants. c, Mock treatment (2 min) of Arabidopsis roots. The volcano plot depicts log2 fold changes (x-axis; abp1-TD1 versus WT) and statistical significance (y axis). Highlighted in red is a subset of significantly regulated P-peptides (FDR < 0.05) which are also significantly regulated in tmk1-1/WT. This shows global hypo-phosphorylation in abp1-TD1 and extensive functional overlap between the two mutants. d, Relative MS intensity for P-peptides pertaining to various Arabidopsis MadB paralogs (MadB1, MadB/At5g20360, MadB2/PHOX2). Auxin (IAA, 100 nM, 2 min) treatment, 4 independent biological replicates, mean ± SD. Asterisks indicate FDR-controlled p-values collated from global phosphoproteomic comparisons as described in Materials and Methods. MadB1S391 (WT versus tmk1-1: no statistics due to low detection, WT versus abp1-TD1: P = 0.0354), MadB2S261 (WT versus tmk1-1: P = 0.1562, WT versus abp1-TD1: P = 1.17E-06), MadBS208 (WT versus tmk1-1: P = 0.0393, WT versus abp1-TD1: P = 0.0035), MadB2T215 (no statistics due to low detection), MadB2S216 (no statistics due to low detection), MadB2S263 (WT versus tmk1-1: P = 0.0926, WT versus abp1-TD1: P = 0.0056). * P ≤ 0.05, ** P ≤ 0.01, ****P ≤ 0.0001. e, Steady-state membrane potentials (MP) and IAA-induced depolarization (in mV) measured in the mature root zone of WT, abp1-c1 and tmk1-1 mutants after 100 nM IAA treatment. Values are means ± SEM (Depolarization amplitudes: WT, n = 9; abp1-c1, n = 10; tmk1-1, n = 3; Membrane potentials: WT, n = 26; abp1-c1, n = 35; tmk1-1, n = 11). f, Auxin sensitivity of abp1-c1 root growth. Data on the graph represent the normalized growth rate of abp1-c1 mutant in comparison to comp-c1 line and WT, mean ± SD (Col-0 DMSO, n = 54; Col-0 3 nM IAA, n = 45; Col-0 5 nM IAA, n = 54; abp1-c1 DMSO, n = 81; abp1-c1 3 nM IAA, n = 90; abp1-c1 5 nM IAA, n = 108; comp-c1 DMSO, n = 90; comp-c1 3 nM IAA, n = 81; comp-c1 5 nM IAA, n = 81). No statistically significant difference in sensitivity of growth rate to auxin was detected with two-way ANOVA. g, Auxin sensitivity of abp1-TD1 root growth. Data on the graph represent the normalized growth rate of abp1-TD1 mutant in comparison to comp-TD1 line and Col-4, mean ± SD (Col-4 DMSO, n = 81; Col-4 3nM IAA, n = 81; Col-4 5 nM IAA, n = 90; abp1-TD1 DMSO, n = 90; abp1-TD1 3 nM IAA, n = 72; abp1-TD1 5 nM IAA, n = 99; comp-TD1 DMSO, n = 81; comp-TD1 3 nM IAA, n = 72; comp-TD1 5 nM IAA, n = 90). No statistically significant difference in sensitivity of growth rate to auxin was detected with two-way ANOVA. h, Cytoplasmic streaming velocity increases with IAA concentration. WT seedlings were treated with 0, 10, 20, 50 and 100 nM IAA for 30 min. Fast-moving particles in epidermal cells of the root elongation zone were recorded. Error bars represent mean ± SD (n = 72 particles from 24 cells in 8 seedlings for each condition). One-way ANOVA (F4,354 = 16.32, P = 2.80E-12) with Dunnett’s multiple comparison tests indicated in the figure. * P ≤ 0.05, **** P ≤ 0.0001. i, Auxin-triggered acceleration of cytoplasmic streaming is not observed in abp1 mutant alleles but is restored in the complemented abp1 lines. Error bars represent mean ± SD (n = 63 particles from 21 cells in 7 seedlings for each condition). Two-way ANOVA (interaction effect: F4,608 = 2.29, P = 0.0585; genotype effect: F4,612 = 5.74, P = 0.0002; treatment effect: F1,612 = 37.26, P = 1.84E-09) with Šidák’s multiple comparison tests indicated in the figure. ** P ≤ 0.01, *** P ≤ 0.001. j. Auxin-triggered acceleration of cytoplasmic streaming is not observed in tmk1 and tmk4 mutants. Unlike tmk4, tmk1 roots have already accelerated cytoplasmic streaming but both mutants are largely auxin-insensitive. Error bars represent mean ± SD (n = 54 particles from 18 cells in 6 seedlings for each condition). Two-way ANOVA (interaction effect: F2,318 = 9.16, P = 0.0001) with Šidák’s multiple comparison tests indicated in the figure. **** P ≤ 0.0001.
Extended Data Fig. 4 ABP1 and TMKs in vasculature formation and regeneration.
a, GUS staining revealed specific upregulation of TMK1::GUS, TMK3::GUS and TMK4::GUS expression around the wound 2 or 4 days after wounding (daw). Representative micrographs from 2 experiments (n = 8 for each). Scale bar, 100 µm. b, GUS staining revealed specific upregulation of ABP1::GUS but not LRR4::GUS expression around the wound. ABP1::GFP-ABP1 confirmed the GUS staining. Representative micrographs from 2 experiments (n = 8 for each). Scale bar, 100 µm. c, Quantification of vasculature regeneration in wounded Arabidopsis stems in abp1-c1 and abp1-TD1 mutants as well as corresponding complemented lines (comp-c1 and comp-TD1) confirms that the vasculature regeneration defects are due to disruption of the ABP1 locus. Total number of samples for each observation, n = 20. d, Exogenous IAA application (green oval shape) on stems triggered the formation of a channel (visualized by toluidine blue (TBO); indicated by white arrow) from this local source to the existing vascular tissue in WT but not abp1-TD1 mutant 6 days after application (daa). Scale bar, 100 µm. e, Quantification of de novo vasculature formation from local auxin source (as visualized by TBO) 4 days after application (4 daa) in abp1-c1 and abp1-TD1 mutants as well as corresponding complemented lines (comp-c1 and comp-TD1) confirms that the vasculature regeneration defects are due to the ABP1 locus disruption. Total number of samples for each observation, n = 20. f, Quantification of de novo vasculature formation from local auxin source (as visualized by TBO) in tmk mutants. tmk4 shows stronger defects followed by tmk3 and tmk1 whereas tmk2 has almost normal vasculature formation. Total number of samples for each observation n = 20.
Extended Data Fig. 5 ABP1 and ABP1(M2X) protein characterization, IAA binding analysis and role in regeneration and auxin canalization.
a, Nearly identical Circular Dichroism (CD) spectra of ABP1(WT) and ABP1(M2X) proteins. This and temperatures of transition midpoints derived from Prometheus thermal stability measurements (ABP1(WT): 58.3 °C, ABP1(M2X): 61.4 °C) suggest similar folding of both protein variants. Error bars represent the mean ± SD; n = 3. b, Western blot detection of ABP1(WT) and ABP1(M2X) proteins in different transgenic lines showing similar size and expression levels. The associated molecular-weight marker is from the same gel and blot. c, DARTS assay on heterologously expressed and purified ABP1(M2X). Purified protein was mixed with different quantities of pronase enzyme mixture and proteolysis was stopped after 30 min. The resulting degraded protein samples were run on SDS–PAGE and blotted for antibody-aided visualization. Pronase-induced proteolysis of tagged ABP1(M2X) was very variable in the presence of 10 μM IAA not supporting any binding of IAA to ABP1(M2X). The intensity profiles are plotted in the graph below the blots. Representative of 3 independent experiments with similar outcome shown. d, Overview table and graphs of all ABP1(M2X) GCI-binding analysis. The potential ligands IAA and benzoic acid were evaluated in serial dilution ranging from 91.5 nM to 200 μM. Albeit ABP1 and ABP1(M2X) could be immobilized at the surface to the same level (compared to Extended Data Fig. 1f), only for ABP1(WT), IAA binding kinetics could be observed while for the analyses using ABP1(M2X) no Kd could be estimated. e, Exogenous IAA application (white oval shape) on DR5rev::GFP stems triggered the formation of a DR5-visualized auxin channels (indicated by white arrow) from this local source 4 days after application (daa) in abp1-c1 transformed with ABP1::GFP- ABP1WT but not in any of the ABP1::GFP-ABP1M2X lines. Scale bar, 100 µm. f, Quantification of de novo vasculature formation from local auxin source in DR5rev::GFP lines as in (e). Total number of samples for each observation n = 40. g, Exogenous IAA application (green oval shape) on stems triggered the formation of a channel (visualized by TBO; indicated by white arrow) from this local source 6 daa in abp1-c1 transformed with ABP1::GFP- ABP1WT but not in any of the ABP1::GFP- ABP1M2X lines. Scale bar, 100 µm. h, Quantification of de novo vasculature formation from (g). Total number of samples for each observation n = 40.
Supplementary information
Supplementary Figure 1
This file contains the raw and uncropped data for every figure, where we show western blot results. The blots are labelled according to the corresponding figure, and the cropped area is indicated.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Friml, J., Gallei, M., Gelová, Z. et al. ABP1–TMK auxin perception for global phosphorylation and auxin canalization. Nature 609, 575–581 (2022). https://doi.org/10.1038/s41586-022-05187-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05187-x
This article is cited by
-
The cell surface is the place to be for brassinosteroid perception and responses
Nature Plants (2024)
-
Switching action modes of miR408-5p mediates auxin signaling in rice
Nature Communications (2024)
-
The new horizon of plant auxin signaling via cell-surface co-receptors
Cell Research (2024)
-
New fluorescent auxin derivatives: anti-auxin activity and accumulation patterns in Arabidopsis thaliana
Plant Growth Regulation (2024)
-
Distant cousins come to ABP1’s rescue
Science China Life Sciences (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.