Abstract
Mandibular teeth and dentitions are features of jawed vertebrates that were first acquired by the Palaeozoic ancestors1,2,3 of living chondrichthyans and osteichthyans. The fossil record currently points to the latter part of the Silurian period4,5,6,7 (around 425 million years ago) as a minimum date for the appearance of gnathostome teeth and to the evolution of growth and replacement mechanisms of mandibular dentitions in the subsequent Devonian period2,8,9,10. Here we provide, to our knowledge, the earliest direct evidence for jawed vertebrates by describing Qianodus duplicis, a new genus and species of an early Silurian gnathostome based on isolated tooth whorls from Guizhou province, China. The whorls possess non-shedding teeth arranged in a pair of rows that demonstrate a number of features found in modern gnathostome groups. These include lingual addition of teeth in offset rows and maintenance of this patterning throughout whorl development. Our data extend the record of toothed gnathostomes by 14 million years from the late Silurian into the early Silurian (around 439 million years ago) and are important for documenting the initial diversification of vertebrates. Our analyses add to mounting fossil evidence that supports an earlier emergence of jawed vertebrates as part of the Great Ordovician Biodiversification Event (approximately 485–445 million years ago).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The tomography slices (bmp), volume renderings (obj) and phylogenetic analyses related files (nex, tnt, tre, xlxs and rft) collected or produced during this study (Supplementary Data 1–6) are available at https://doi.org/10.6084/m9.figshare.20366757.v1. The ZooBank LSID code for this publication is urn:lsid:zoobank.org:pub:F2565F74-5E03-482D-9FDA-72705EB36966. The ZooBank LSID code for the new genus Qianodus is urn:lsid:zoobank.org:act:A072F8AA-1AAF-4837-9F70-F98A4113E606. The ZooBank LSID code for the new species Q. duplicis is urn:lsid:zoobank.org:act:D85084C9-36CC-471E-9E52-51DCB3429618. The examined tooth whorl specimens IVPP V26641–V26663 are available on request from the Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China.
References
Donoghue, P. C. & Rücklin, M. The ins and outs of the evolutionary origin of teeth. Evol. Dev. 18, 19–30 (2016).
Smith, M. M. Vertebrate dentitions at the origin of jaws: when and how pattern evolved. Evol. Dev. 5, 394–413 (2003).
Vaškaninová, V. et al. Marginal dentition and multiple dermal jawbones as the ancestral condition of jawed vertebrates. Science 369, 211–216 (2020).
Chen, D., Blom, H., Sanchez, S., Tafforeau, P. & Ahlberg, P. E. The stem osteichthyan Andreolepis and the origin of tooth replacement. Nature 539, 237–241 (2016).
Chen, D. et al. Development of cyclic shedding teeth from semi-shedding teeth: the inner dental arcade of the stem osteichthyan Lophosteus. R. Soc. Open Sci. 4, 161084 (2017).
Choo, B., Zhu, M., Zhao, W. & Jia, L. The largest Silurian vertebrate and its palaeoecological implications. Sci. Rep. 4, 5242 (2014).
Zhu, M. et al. The oldest articulated osteichthyan reveals mosaic gnathostome characters. Nature 458, 469–474 (2009).
Denison, R. H. Placodermi Vol. 2 (Gustav Fischer, 1978).
Ginter, M., Hampe, O., Duffin, C. J. & Schultze, H. Handbook of Paleoichthyology Volume 3D. Chondrichthyes. Paleozoic Elasmobranchii: Teeth (Dr Friedrich Pfeil, 2010).
Rücklin, M. et al. Development of teeth and jaws in the earliest jawed vertebrates. Nature 491, 748–751 (2012).
Brazeau, M. D. & Friedman, M. The origin and early phylogenetic history of jawed vertebrates. Nature 520, 490–497 (2015).
Sansom, I. J. & Andreev, P. S. in Evolution and Development of Fishes (eds Johanson, Z. et al.) 59–70 (Cambridge Univ. Press, 2019).
Thanh, T.-D., Phuong, T. H., Boucot, A. J., Goujet, D. & Janvier, P. Silurian vertebrates from Central Vietnam. C. R. Acad. Sci. Paris 324, 1023–1030 (1997).
Zhao, W.-J. et al. A review of Silurian fishes from north-western Hunan, China and related biostratigraphy. Acta Geol. Pol. 68, 475–486 (2018).
Zhu, M. et al. A Silurian maxillate placoderm illuminates jaw evolution. Science 354, 334–336 (2016).
Burrow, C. J. & Rudkin, D. Oldest near-complete acanthodian: the first vertebrate from the Silurian Bertie Formation Konservat-Lagerstätte, Ontario. PLoS ONE 9, e104171 (2014).
Brazeau, M. D. A revision of the anatomy of the Early Devonian jawed vertebrate Ptomacanthus anglicus Miles. Palaeontology 55, 355–367 (2012).
Burrow, C. J., Davidson, R. G., Den Blaauwen, J. L. & Newman, M. J. Revision of Climatius reticulatus Agassiz, 1844 (Acanthodii, Climatiidae), from the Lower Devonian of Scotland, based on new histological and morphological data. J. Vertebr. Paleontol. 35, e913421 (2015).
Burrow, C. J., Newman, M., Den Blaauwen, J., Jones, R. & Davidson, R. The Early Devonian ischnacanthiform acanthodian Ischnacanthus gracilis (Egerton, 1861) from the Midland Valley of Scotland. Acta Geol. Pol. 68, 335–362 (2018).
Maisey, J. et al. in Evolution and Development of Fishes (eds Johanson, Z. et al.) 87–109 (Cambridge Univ. Press, 2019).
Burrow, C. J. & Simpson, A. J. A new ischnacanthid acanthodian from the Late Silurian (Ludlow, ploeckensis Zone) Jack Formation, north Queensland. Mem. Queensl. Mus. 38, 383–396 (1995).
Gross, W. Mundzähne und Hautzähne der Acanthodier und Arthrodiren. Palaeontogr. Abt. A 1–2, 1–40 (1957).
Martínez-Pérez, C. et al. Vascular structure of the earliest shark teeth. Acta Geol. Pol. 68, 335–362 (2018).
Coates, M. I. et al. An early chondrichthyan and the evolutionary assembly of a shark body plan. Proc. R. Soc. B 285, 20172418 (2018).
Andreev, P. S. et al. The systematics of the Mongolepidida (Chondrichthyes) and the Ordovician origins of the clade. PeerJ 4, e1850 (2016).
Andreev, P. S. et al. Early Silurian chondrichthyans from the Tarim Basin (Xinjiang, China). PLoS ONE 15, e0228589 (2020).
Keating, J. N., Marquart, C. L. & Donoghue, P. C. Histology of the heterostracan dermal skeleton: insight into the origin of the vertebrate mineralised skeleton. J. Morph. 276, 657–680 (2015).
Dearden, R. P., Stockey, C. & Brazeau, M. D. The pharynx of the stem-chondrichthyan Ptomacanthus and the early evolution of the gnathostome gill skeleton. Nat. Commun. 10, 2050 (2019).
Gegenbaur, C. Grundriss der Vergleichenden Anatomie (Wilhelm Engelmann, 1874).
Huxley, T. H. On the application of the laws of evolution to the arrangement of the Vertebrata, and more particularly of the Mammalia. Proc. Sci. Meet. Zool. Soc. Lond. 1880, 649–662 (1880).
Rücklin, M. et al. Acanthodian dental development and the origin of gnathostome dentitions. Nat. Ecol. Evol. 5, 919–926 (2021).
Maisey, J. G., Turner, S., Naylor, G. J. & Miller, R. F. Dental patterning in the earliest sharks: implications for tooth evolution. J. Morph. 275, 586–596 (2014).
Underwood, C., Johanson, Z. & Smith, M. M. Cutting blade dentitions in squaliform sharks form by modification of inherited alternate tooth ordering patterns. R. Soc. Open Sci. 3, 160385 (2016).
Underwood, C. J. et al. Development and evolution of dentition pattern and tooth order in the skates and rays (Batoidea; Chondrichthyes). PLoS ONE 10, e0122553 (2015).
Smith, M. M. & Coates, M. I. in Major Events in Early Vertebrate Evolution (ed. Ahlberg, P. E.) 223–240 (Taylor & Francis, 2001).
Coates, M. & Sequeira, S. A new stethacanthid chondrichthyan from the Lower Carboniferous of Bearsden, Scotland. J. Vertebr. Paleontol. 21, 438–459 (2001).
Zangerl, R. & Case, G. Cobelodus aculeatus (Cope), an anacanthous shark from Pennsylvanian black shales of North America. Palaeontogr. Abt. A 154, 107–157 (1976).
Andrews, M., Long, J., Ahlberg, P., Barwick, R. & Campbell, K. The structure of the sarcopterygian Onychodus jandemarrai n. sp. from Gogo, Western Australia: with a functional interpretation of the skeleton. Earth. Env. Sci. Trans. R. Soc. Edinb. 96, 197–307 (2005).
Blais, S. A., MacKenzie, L. A. & Wilson, M. V. Tooth-like scales in Early Devonian eugnathostomes and the ‘outside-in’ hypothesis for the origins of teeth in vertebrates. J. Vertebr. Paleontol. 31, 1189–1199 (2011).
Frey, L. et al. The early elasmobranch Phoebodus: phylogenetic relationships, ecomorphology and a new time-scale for shark evolution. Proc. R. Soc. B 286, 20191336 (2019).
Hu, Y., Lu, J. & Young, G. C. New findings in a 400 million-year-old Devonian placoderm shed light on jaw structure and function in basal gnathostomes. Sci. Rep. 7, 7813 (2017).
Hu, Y.-Z., Young, G., Burrow, C., Zhu, Y.-A. & Lu, J. High resolution XCT scanning reveals complex morphology of gnathal elements in an Early Devonian arthrodire. Palaeoworld 28, 525–534 (2019).
Doeland, M., Couzens, A. M., Donoghue, P. C. & Rücklin, M. Tooth replacement in early sarcopterygians. R. Soc. Open Sci. 6, 191173 (2019).
Zhu, M. & Yu, X. in Recent Advances in the Origin and Early Radiation of Vertebrates (eds Arratia, G. et al.) 271–286 (Dr. Friedrich Pfeil, 2004).
Burrow, C. J., Newman, M. J., Davidson, R. G. & den Blaauwen, J. L. Redescription of Parexus recurvus, an Early Devonian acanthodian from the Midland Valley of Scotland. Alcheringa 37, 392–414 (2013).
Dearden, R. P. & Giles, S. Diverse stem-chondrichthyan oral structures and evidence for an independently acquired acanthodid dentition. R. Soc. Open Sci. 8, 210822 (2021).
Zangerl, R. & Case, G. R. Iniopterygia, a New Order of Chondrichthyan Fishes from the Pennsylvanian of North America (Field Museum of Natural History, 1973).
Andreev, P. S. et al. Upper Ordovician chondrichthyan‐like scales from North America. Palaeontology 58, 691–704 (2015).
Servais, T. & Harper, D. A. The great Ordovician biodiversification event (GOBE): definition, concept and duration. Lethaia 51, 151–164 (2018).
Wang, C.-C. Joint iterative fast projection matching for fully automatic marker-free alignment of nano-tomography reconstructions. Sci. Rep. 10, 7330 (2020).
Wang, Y. et al. Development and applications of paleontological computed tomography. Vertebr. PalAsiat. 57, 84–92 (2019).
Goloboff, P. A. & Catalano, S. A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32, 221–238 (2016).
Brazeau, M. et al. Endochondral bone in an Early Devonian ‘placoderm’ from Mongolia. Nat. Ecol. Evol. 4, 1477–1484 (2020).
Dearden, R. P. The Anatomy and Evolution of “Acanthodian” Stem-chondrichthyans. PhD thesis, Imperial College London (2018).
Giles, S., Friedman, M. & Brazeau, M. D. Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome. Nature 520, 82–85 (2015).
King, B., Qiao, T., Lee, M. S., Zhu, M. & Long, J. A. Bayesian morphological clock methods resurrect placoderm monophyly and reveal rapid early evolution in jawed vertebrates. Syst. Biol. 66, 499–516 (2017).
Qiao, T., King, B., Long, J. A., Ahlberg, P. E. & Zhu, M. Early gnathostome phylogeny revisited: multiple method consensus. PLoS ONE 11, e0163157 (2016).
Zhu, Y.-a, Lu, J. & Zhu, M. Reappraisal of the Silurian placoderm Silurolepis and insights into the dermal neck joint evolution. R. Soc. Open Sci. 6, 191181 (2019).
Bapst, D. W. paleotree: an R package for paleontological and phylogenetic analyses of evolution. Methods Ecol. Evol. 3, 803–807 (2012).
Botella, H., Manzanares, E., Ferrón, H. & Martínez-Pérez, C. Obruchevacanthus ireneae gen. et sp. nov., a new ischnacanthiform (Acanthodii) from the Lower Devonian of Spain. Paleontol. J. 48, 1067–1076 (2014).
Gagnier, P.-Y. & Wilson, M. V. An unusual acanthodian from northern Canada: revision of Brochoadmones milesi. Mod. Geol. 20, 235–252 (1996).
Jarvik, E. Middle and Upper Devonian Porolepiformes from East Greenland with special reference to Glyptolepis groenlandica n. sp. and a discussion on the structure of the head in the Porolepiformes. Medd. Grønl. 187, 1–307 (1972).
Long, J. New palaeoniscoid fishes from the Late Devonian and Early Carboniferous of Victoria. Mem. Assoc. Australas. Palaeontol. 7, 1–64 (1988).
Miles, R. S. Articulated acanthodian fishes from the Old Red Sandstone of England: with a review of the structure and evolution of the acanthodian shoulder-girdle. Bull. Br. Mus. Nat. Hist. Geol. 24, 111–213 (1973).
Mondéjar‐Fernández, J., Friedman, M. & Giles, S. Redescription of the cranial skeleton of the Early Devonian (Emsian) sarcopterygian Durialepis edentatus Otto (Dipnomorpha, Porolepiformes). Pap. Palaeontol. 7, 789–806 (2020).
Qu, Q., Sanchez, S., Blom, H., Tafforeau, P. & Ahlberg, P. E. Scales and tooth whorls of ancient fishes challenge distinction between external and oral ‘teeth’. PLoS ONE 8, e71890 (2013).
Vergoossen, J. Late Silurian fish microfossils from Helvetesgraven, Skåne (southern Sweden) (I). Geol. Mijnbouw 78, 267–280 (1999).
Wang, N.-Z. Microremains of agnathans and fishes from Lower Devonian of central Guangxi with correlation of Lower Devonian between central Guangxi and eastern Yunnan, South China. Acta Palaeontol. Sin. 31, 280–303 (1992).
Acknowledgements
We thank J.-C. Cai for the field work, Y.-M. Hou for the acquisition of the micro-computed tomography X-ray data, Y. Hwu and Y.-T. Weng for performing and assisting with the synchrotron X-ray analyses and Y. Z. Hu for her comments and advice during the preparation of tooth-whorl volume renderings in Drishti. This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA19050102 and XDB26000000), the National Natural Science Foundation of China (42130209), the Key Research Program of Frontier Sciences, CAS (QYZDJ-SSW-DQC002), an Open Project Grant of the Key Laboratory of Vertebrate Evolution and Human Origins, IVPP, CAS (LVEHO19001), MOST 108-2116-M-213-001 (Taiwan), Chinese Postdoctoral Science Foundation grant (2019M663440) and the National Synchrotron Radiation Research Center, Taiwan (beamtime projects 2019-3-083-1 and 2019-3-185-1).
Author information
Authors and Affiliations
Contributions
Research design: M.Z., P.S.A. and I.J.S. Fieldwork and sample collection: M.Z., W.Z., Q.L., J.W., L.J., T.Q. and L.P. Data processing: Q.L., P.S.A., L.P., J.W. and M.Z. Synchrotron X‐ray tomography analyses: P.S.A. and C.-C.W. Manuscript text and figure preparation: P.S.A., I.J.S., Q.L., J.W. and M.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Matt Friedman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
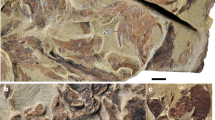
Extended Data Fig. 1 Morphology of Qianodus tooth whorls.
(a–c, i, j, m) Scanning electron microscopy, volume renderings of (g, h) synchrotron and (d–f) microcomputed X-ray tomography datasets and (k, l, n) light microscopy. (a) Lateral (mesial or distal) view of a heavily abraded tooth whorl (IVPP V26648). (b) Mesial view of a tooth whorl (IVPP V26649). (c) Latero-posterior view of a tooth whorl (IVPP V26652). (d–f) Tooth whorl (IVPP V26647) in (d) labial, (e) mesial and (f) basal views. (g, h) Incomplete tooth (IVPP V26645) whorl in (g) mesial and (h) basal views. (i) Lateral view of a tooth whorl (IVPP V26654) with a flared-out base. (j–n) Two complete whorls with 6 recognizable primary teeth in (j, k) lateral (distal and mesial) (IVPP V26650), (m) oral (IVPP V26651) and (l, n) basal (IVPP V26650, 51) views. at, accessory teeth; la, labial, li, lingual; pt, primary teeth. Scale bars, 0.5 mm.
Extended Data Fig. 2 Internal structure of Qianodus tooth whorls.
(a, g) Nomarski DIC optical microscopy and (b–f, h) volume renderings of synchrotron X-ray tomography datasets. (a) Longitudinal thin section through a whorl with partially preserved teeth IVPP V26653. (b) Longitudinal virtual slice through the progenitor tooth row of the holotype IVPP V26641. (c) Horizontal virtual slice through the holotype IVPP V26641 at the level of tooth. (d) Transverse virtual slice through a partially preserved whorl IVPP V26645. (e) Basal view of IVPP V26641 with highlighted compact tissue of the base. (f) Volume rendering of radiotransparent structures inside a tooth whorl fragment (IVPP V26646) shown in oral view. (g) Longitudinal thin section and (h) longitudinal virtual section through IVPP V26646. at, accessory teeth; ct, compact tissue; la, labial; li, lingual; p, tooth pulp; pt, primary teeth; st, spongiose tissue; stc, spongiose tissue canals; wbc, whorl base crest. Scale bars, 0.5 mm.
Extended Data Fig. 3 Comparison of Qianodus tooth whorls with the whorl-based dentition of the stem chondrichthyan Doliodus problematicus.
(a) Qianodus tooth whorls at a late stage of development (from top to bottom IVPP V26652, V26641, V26649, V26647, V26655 and V26648). (b) Tooth whorls of the lower left jaw ramus of Doliodus at positions 1 to 9 (P1–9) (adapted from Maisey et al.32).
Extended Data Fig. 4 Phylogenetic position of Qianodus within early jawed vertebrates.
50 percent majority-rule consensus tree from a parsimony analysis of 105 taxa and 294 characters. Tree time-adjusted using minimum branch length scaling. Taxon and tree root ages sourced from King et al.56 and other studies (for a full list of studies, see Supplementary Table 1). Colour coding of cladogram branches: jawless stem gnathostomes (purple), ‘placoderms’ (black), Osteichthyes (green), stem Chondrichthyes (ochre), crown Chondrichthyes (blue). Pie charts represent Markov k-state 1 likelihood values for tooth whorl/dentition characters at select internal nodes. Circles show character states at terminal nodes. Character numbers shown in parentheses.
Extended Data Fig. 5 Results of the parsimony analysis described in the Methods section and in Extended Data Fig. 4.
(a) 50% majority-rule consensus and (b) strict consensus tree topologies. Squares in (a) depict most-parsimonious character state reconstructions at select internal nodes (character numbers shown in parentheses). Numbers at internal branches represent bootstrap values of 50 percent and above.
Supplementary information
Supplementary Information
(1) Geological setting and biostratigraphy of the Rongxi Formation. (2) Character list for the phylogenetic analysis. (3) Supplementary Table 1. (4) Supplementary references. Supplementary Data files 1–6 are available online at https://doi.org/10.6084/m9.figshare.20366757.v1. These include the tomography slices (bmp), volume renderings (obj) and phylogenetic analyses related files (nex, tnt, tre, xlxs and rft) collected or produced during this study.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andreev, P.S., Sansom, I.J., Li, Q. et al. The oldest gnathostome teeth. Nature 609, 964–968 (2022). https://doi.org/10.1038/s41586-022-05166-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05166-2
This article is cited by
-
How China is capturing attention with landmark research
Nature (2023)
-
How did Jawed Vertebrates Originate and Rise?
Journal of Earth Science (2023)
-
Spiny chondrichthyan from the lower Silurian of South China
Nature (2022)
-
In praise of research in fundamental biology
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.