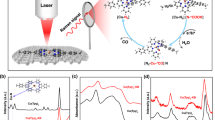
Abstract
Transition metal hydrides (M-H) are ubiquitous intermediates in a wide range of enzymatic processes and catalytic reactions, playing a central role in H+/H2 interconversion1, the reduction of CO2 to formic acid (HCOOH)2 and in hydrogenation reactions. The facile formation of M-H is a critical challenge to address to further improve the energy efficiency of these reactions. Specifically, the easy electrochemical generation of M-H using mild proton sources is key to enable high selectivity versus competitive CO and H2 formation in the CO2 electroreduction to HCOOH, the highest value-added CO2 reduction product3. Here we introduce a strategy for electrocatalytic M-H generation using concerted proton–electron transfer (CPET) mediators. As a proof of principle, the combination of a series of CPET mediators with the CO2 electroreduction catalyst [MnI(bpy)(CO)3Br] (bpy = 2,2′-bipyridine) was investigated, probing the reversal of the product selectivity from CO to HCOOH to evaluate the efficiency of the manganese hydride (Mn-H) generation step. We demonstrate the formation of the Mn-H species by in situ spectroscopic techniques and determine the thermodynamic boundary conditions for this mechanism to occur. A synthetic iron–sulfur cluster is identified as the best CPET mediator for the system, enabling the preparation of a benchmark catalytic system for HCOOH generation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study (catalytic activity tests, cyclic voltammograms, NMR, UV–Vis and IRSEC spectra) are available within the paper and its Supplementary Information files.
References
Bullock, R. M. & Helm, M. L. Molecular electrocatalysts for oxidation of hydrogen using Earth-abundant metals: shoving protons around with proton relays. Acc. Chem. Res. 48, 2017–2026 (2015).
Francke, R., Schille, B. & Roemelt, M. Homogeneously catalyzed electroreduction of carbon dioxide—methods, mechanisms, and catalysts. Chem. Rev. 118, 4631–4701 (2018).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Elgrishi, N., Kurtz, D. A. & Dempsey, J. L. Reaction parameters influencing cobalt hydride formation kinetics: implications for benchmarking H2-evolution catalysts. J. Am. Chem. Soc. 139, 239–244 (2017).
Huang, T., Rountree, E. S., Traywick, A. P., Bayoumi, M. & Dempsey, J. L. Switching between stepwise and concerted proton-coupled electron transfer pathways in tungsten hydride activation. J. Am. Chem. Soc. 140, 14655–14669 (2018).
Kurtz, D. A. et al. Redox-induced structural reorganization dictates kinetics of cobalt(III) hydride formation via proton-coupled electron transfer. J. Am. Chem. Soc. 143, 3393–3406 (2021).
Noh, H. et al. Redox-mediator-assisted electrocatalytic hydrogen evolution from water by a molybdenum sulfide-functionalized metal–organic framework. ACS Catal. 8, 9848–9858 (2018).
Rausch, B., Symes, M. D. & Cronin, L. A bio-inspired, small molecule electron-coupled-proton buffer for decoupling the half-reactions of electrolytic water splitting. J. Am. Chem. Soc. 135, 13656–13659 (2013).
Dutta, A., Appel, A. M. & Shaw, W. J. Designing electrochemically reversible H2 oxidation and production catalysts. Nat. Rev. Chem. 2, 244–252 (2018).
Smith, N. E., Bernskoetter, W. H. & Hazari, N. The role of proton shuttles in the reversible activation of hydrogen via metal–ligand cooperation. J. Am. Chem. Soc. 141, 17350–17360 (2019).
Badalyan, A. & Stahl, S. S. Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators. Nature 535, 406–410 (2016).
McLoughlin, E. A., Armstrong, K. C. & Waymouth, R. M. Electrochemically regenerable hydrogen atom acceptors: mediators in electrocatalytic alcohol oxidation reactions. ACS Catal. 10, 11654–11662 (2020).
Galvin, C. M. & Waymouth, R. M. Electron-rich phenoxyl mediators improve thermodynamic performance of electrocatalytic alcohol oxidation with an iridium pincer complex. J. Am. Chem. Soc. 142, 19368–19378 (2020).
Chalkley, M. J., Garrido-Barros, P. & Peters, J. C. A molecular mediator for reductive concerted proton–electron transfers via electrocatalysis. Science 369, 850–854 (2020).
Anson, C. W. & Stahl, S. S. Cooperative electrocatalytic O2 reduction involving Co(salophen) with p-hydroquinone as an electron–proton transfer mediator. J. Am. Chem. Soc. 139, 18472–18475 (2017).
Bourrez, M., Molton, F., Chardon-Noblat, S. & Deronzier, A. [Mn(bipyridyl)(CO)3Br]: an abundant metal carbonyl complex as efficient electrocatalyst for CO2 reduction. Angew. Chem. Int. Ed. Engl. 50, 9903–9906 (2011).
Takeda, H., Koizumi, H., Okamoto, K. & Ishitani, O. Photocatalytic CO2 reduction using a Mn complex as a catalyst. Chem. Commun. 50, 1491–1493 (2014).
Wang, X. et al. Site-isolated manganese carbonyl on bipyridine-functionalities of periodic mesoporous organosilicas: efficient CO2 photoreduction and detection of key reaction intermediates. Chem. Sci. 8, 8204–8213 (2017).
Rønne, M. H. et al. Ligand-controlled product selectivity in electrochemical carbon dioxide reduction using manganese bipyridine catalysts. J. Am. Chem. Soc. 142, 4265–4275 (2020).
Bhattacharya, M., Sebghati, S., VanderLinden, R. T. & Saouma, C. T. Toward combined carbon capture and recycling: addition of an amine alters product selectivity from CO to formic acid in manganese catalyzed reduction of CO2. J. Am. Chem. Soc. 142, 17589–17597 (2020).
Saouma, C. T., Morris, W. D., Darcy, J. W. & Mayer, J. M. Protonation and proton-coupled electron transfer at S-ligated [4Fe-4S] clusters. Chem. Eur. J. 21, 9256–9260 (2015).
Tilset, M. & Parker, V. D. Solution homolytic bond dissociation energies of organotransition-metal hydrides. J. Am. Chem. Soc. 111, 6711–6717 (1989).
Senger, M. et al. Proton-coupled reduction of the catalytic [4Fe-4S] cluster in [FeFe]-hydrogenases. Angew. Chem. Int. Ed. Engl. 56, 16503–16506 (2017).
Camba, R. et al. Mechanisms of redox-coupled proton transfer in proteins: role of the proximal proline in reactions of the [3Fe-4S] cluster in Azotobacter vinelandii ferredoxin I. Biochemistry 42, 10589–10599 (2003).
Albers, A. et al. Fast proton-coupled electron transfer observed for a high-fidelity structural and functional [2Fe–2S] Rieske model. J. Am. Chem. Soc. 136, 3946–3954 (2014).
Kennepohl, P. & Solomon, E. I. Electronic structure contributions to electron-transfer reactivity in iron−sulfur active sites: 3. Kinetics of electron transfer. Inorg. Chem. 42, 696–708 (2003).
Sigfridsson, E., Olsson, M. H. M. & Ryde, U. Inner-sphere reorganization energy of iron−sulfur clusters studied with theoretical methods. Inorg. Chem. 40, 2509–2519 (2001).
Jordan, R. F. & Norton, J. R. in Mechanistic Aspects of Inorganic Reactions (eds Rorabacher, D. B. & Endicott, J. F.) ACS Symposium Series Vol. 198, Ch. 17, 403–423 (American Chemical Society, 1982).
Franco, F. et al. Local proton source in electrocatalytic CO2 reduction with [Mn(bpy–R)(CO)3Br] complexes. Chem. Eur. J. 23, 4782–4793 (2017).
Cotton, F. A., Down, J. L. & Wilkinson, G. Infrared spectra of manganese carbonyl hydride and deuteride. J. Chem. Soc. 833–837 (1959).
Machan, C. W., Sampson, M. D., Chabolla, S. A., Dang, T. & Kubiak, C. P. Developing a mechanistic understanding of molecular electrocatalysts for CO2 reduction using infrared spectroelectrochemistry. Organometallics 33, 4550–4559 (2014).
Riplinger, C., Sampson, M. D., Ritzmann, A. M., Kubiak, C. P. & Carter, E. A. Mechanistic contrasts between manganese and rhenium bipyridine electrocatalysts for the reduction of carbon dioxide. J. Am. Chem. Soc. 136, 16285–16298 (2014).
Mayer, J. M. Understanding hydrogen atom transfer: from bond strengths to Marcus theory. Acc. Chem. Res. 44, 36–46 (2011).
Waldie, K. M., Ostericher, A. L., Reineke, M. H., Sasayama, A. F. & Kubiak, C. P. Hydricity of transition-metal hydrides: thermodynamic considerations for CO2 reduction. ACS Catal. 8, 1313–1324 (2018).
Ceballos, B. M. & Yang, J. Y. Directing the reactivity of metal hydrides for selective CO2 reduction. Proc. Natl Acad. Sci. USA 115, 12686–12691 (2018).
Parsell, T. H., Yang, M.-Y. & Borovik, A. S. C−H Bond cleavage with reductants: re-investigating the reactivity of monomeric MnIII/IV−oxo complexes and the role of oxo ligand basicity. J. Am. Chem. Soc. 131, 2762–2763 (2009).
Pattanayak, S. et al. Spectroscopic and reactivity comparisons of a pair of bTAML complexes with FeV═O and FeIV═O units. Inorg. Chem. 56, 6352–6361 (2017).
Qiu, G. & Knowles, R. R. Rate–driving force relationships in the multisite proton-coupled electron transfer activation of ketones. J. Am. Chem. Soc. 141, 2721–2730 (2019).
Madsen, M. R. et al. Promoting selective generation of formic acid from CO2 using Mn(bpy)(CO)3Br as electrocatalyst and triethylamine/isopropanol as additives. J. Am. Chem. Soc. 143, 20491–20500 (2021).
Smith, P. T., Weng, S. & Chang, C. J. An NADH-inspired redox mediator strategy to promote second-sphere electron and proton transfer for cooperative electrochemical CO2 reduction catalyzed by iron porphyrin. Inorg. Chem. 59, 9270–9278 (2020).
Acknowledgements
We thank L. Grunwald and A. Mouchfiq for technical assistance and preliminary studies, and R. Verel for assistance with NMR studies. We acknowledge funding from Agence Nationale pour la Recherche, ANR Jeune Chercheur-Jeune Chercheuse ANR-17-CE05-0021 (S.D., V.M.); European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 853064 (S.D., F.M., V.M.)); and Swiss National Science Foundation (SNSF) project funding (grant no. 200021_197153 / 1 (V.M.)).
Author information
Authors and Affiliations
Contributions
S.D. and V.M. conceptualized the study and were responsible for the methodology. S.D., F.M. and E.B. undertook the investigations. V.M. was responsible for funding acquisition and supervision. M.F. and V.M. administered the project. S.D., F.M. and V.M. wrote the original draft. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary data and discussion, figures, tables and references.
Rights and permissions
About this article
Cite this article
Dey, S., Masero, F., Brack, E. et al. Electrocatalytic metal hydride generation using CPET mediators. Nature 607, 499–506 (2022). https://doi.org/10.1038/s41586-022-04874-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04874-z
This article is cited by
-
Metal surfaces catalyse polarization-dependent hydride transfer from H2
Nature Catalysis (2023)
-
Surface passivation for highly active, selective, stable, and scalable CO2 electroreduction
Nature Communications (2023)
-
Direct OC-CHO coupling towards highly C2+ products selective electroreduction over stable Cu0/Cu2+ interface
Nature Communications (2023)
-
Acidic CO2-to-HCOOH electrolysis with industrial-level current on phase engineered tin sulfide
Nature Communications (2023)
-
Tuning molecular electrophilicity on Cu catalysts to steer CO2 electroreduction selectivity
Science China Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.