Abstract
Palaeospondylus gunni, from the Middle Devonian period, is one of the most enigmatic fossil vertebrates, and its phylogenetic position has remained unclear since its discovery in Scotland in 1890 (ref. 1). The fossil’s strange set of morphological features has made comparisons with known vertebrate morphotype diversity difficult. Here we use synchrotron radiation X-ray micro-computed tomography to show that Palaeospondylus was a sarcopterygian, and most probably a stem-tetrapod. The skeleton of Palaeospondylus consisted solely of endoskeletal elements in which hypertrophied chondrocyte cell lacunae, osteoids and a small fraction of perichondral bones developed. Despite the complete lack of teeth and dermal bones, the neurocranium of Palaeospondylus resembles those of stem-tetrapod Eusthenopteron2 and Panderichthys3, and phylogenetic analyses place Palaeospondylus in between them. Because the unique features of Palaeospondylus, such as the cartilaginous skeleton and the absence of paired appendages, are present in the larva of crown tetrapods, our study highlights an unanticipated heterochronic evolution at the root of tetrapods.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Series of CT images of NSMPV 24679 with voxel sizes of 6.63, 2.74 and 1.46 µm (in 8-bit TIFF format; media IDs: 000435207, 000435203 and 000434543, respectively) and surface files of the same specimen with a voxel size of 6.63 µm (in STL format) have been deposited at MorphoSource at https://www.morphosource.org/projects/000433576. A series of CT images of NSMPV 24,678 with a voxel size of 6.63 µm (in 8-bit TIFF format; media ID: 000435213) and surface files of the same specimen with a voxel size of 6.63 µm (in STL format) have been deposited at MorphoSource at https://www.morphosource.org/projects/000435209. MrBayes and PAUP* executable files (in NEXUS format) are presented in the Supplementary Information (Supplementary Data 1–4).
Code availability
The filtered back-projection algorithm26 used for reconstitutions of SRXµCT data is available online at http://www-bl20.spring8.or.jp/xct/index-e.html.
References
Traquair, R. H. On the fossil fishes at Achanarras Quarry, Caithness. Ann. Mag. Nat. Hist. 6, 479–486 (1890).
Jarvik, E. Basic Structure and Evolution of Vertebrates Vol. I (Academic Press, 1980).
Ahlberg, P. E., Clack, J. A. & Lukševičs, E. Rapid braincase evolution between Panderichthys and the earliest tetrapods. Nature 381, 61–64 (1996).
Trewin, N. H. Palaeoecology and sedimentology of the Archanarras fish bed of the Middle Old Red Sandstone, Scotland. Trans. R. Soc. Edinb. Earth Sci. 77, 21–46 (1986).
Sollas, W. J. & Sollas, I. B. J. An account of the Devonian fish Palaeospondylus gunni Traquair. Philos. Trans. R. Soc. Lond. B 196, 267–294 (1904).
den Blaauwen, J., Barwick, R. E. & Campbell, K. S. W. Structure and function of the tooth plates of the Devonian lungfish Dipterus valenciennesi from Caithness and the Orkney Islands. Rec. West. Aust. Mus. 23, 91–113 (2005).
Traquair, R. H. A still further contribution to our knowledge of Palaeospondylus gunni. Proc. R. Soc. Edinb. 12, 312–321 (1893).
Dean, B. The so-called Devonian lamprey, Palaeospondylus gunni: with notes on the systematic arrangement of the fish-like vertebrates. Mem. N. Y. Acad. Sci. 2, 1–32 (1900).
Johanson, Z. et al. Questioning hagfish affinities of the enigmatic Devonian vertebrate Palaeospondylus. R. Soc. Open Sci. 4, 170214 (2017).
Keating, J. N. & Donoghue, P. C. Histology and affinity of anaspids, and the early evolution of the vertebrate dermal skeleton. Proc. R. Soc. B 283, 20152917 (2016).
Johanson, Z., Kearsley, A., den Blaauwen, J., Newman, M. & Smith, M. M. Ontogenetic development of an exceptionally preserved Devonian cartilaginous skeleton. J. Exp. Zool. B 318B, 50–58 (2012).
de Pinna, M. C. C. Concepts and tests of homology in the cladistic paradigm. Cladistics 7, 367–394 (1991).
Moy-Thomas, J. A. The Devonian fish Palaeospondylus gunni Traquair. Philos. Trans. R. Soc. Lond. B 230, 391–413 (1940).
Higuchi, S. et al. Inner ear development in cyclostomes and evolution of the vertebrate semicircular canals. Nature 565, 347–350 (2018).
Dutel, H. et al. Neurocranial development of the coelacanth and the evolution of the sarcopterygian head. Nature 569, 556–559 (2019).
Campbell, K. S. W., Barwick, R. E. & Senden, T. Development of the posterior endocranium of the Devonian dipnoan Griphognathus whitei. J Vertebr. Paleontol. 32, 781–798 (2012).
Cloutier, R. et al. Elpistostege and the origin of the vertebrate hand. Nature 579, 549–554 (2020).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Swofford, D. L. PAUP*. Phylogenetic Analyses Using Parsimony (*And Other Methods) (Sinauer Associates, 2003).
Coates, M. I. The Devonian tetrapod Acanthostega gunnari Jarvik: postcranial anatomy, basal tetrapod interrelationships and patterns of skeletal evolution. Trans. R. Soc. Edinb. Earth Sci. 87, 363–421 (1996).
Schultze, H. P. 1984. Juvenile specimens of Eusthenopteron foordi Whiteaves, 1881 (osteolepiform rhipidistian, Pisces) from the Late Devonian of Miguasha, Quebec, Canada. J. Vertebr. Paleontol. 4, 1–16 (1984).
Cloutier, R. The fossil record of fish ontogenies: insights into developmental patterns and processes. Sem. Cell Dev. Biol. 21, 400–413 (2010).
Schoch, R. R. & Witzmann, F. Bystrow's paradox: gills, fossils, and the fish-to-tetrapod transition. Acta Zool. 92, 251–265 (2011).
Sanchez, S., Tafforeau, P., Clack, J. A. & Ahlberg, P. E. Life history of the stem tetrapod Acanthostega revealed by synchrotron microtomography. Nature 537, 408–411 (2016).
Niedźwiedzki, G., Szrek, P., Narkiewicz, K., Narkiewicz, M. & Ahlberg, P. E. Tetrapod trackways from the early Middle Devonian period of Poland. Nature 463, 43–48 (2010).
Uesugi, K. et al. Development of fast (sub-minute) micro-tomography. AIP Conf. Proc. 1266, 47–50 (2010).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676–682 (2012).
Limaye, A. Drishti: a volume exploration and presentation tool. Proc. SPIE 8506, 85060X (2012).
Hu, Y., Limaye, A. & Lu, J. Three-dimensional segmentation of computed tomography data using Drishti Paint: new tools and developments. R. Soc. Open Sci. 7, 201033201033 (2020).
Hirasawa, T. et al. Development of the pectoral lobed fin in the Australian lungfish Neoceratodus forsteri. Front. Ecol. Evol. 9, 679633 (2021).
Kemp, A. The embryological development of the Queensland lungfish, Neoceratodus forsteri (Krefft). Mem. Queensl. Mus. 20, 553–597 (1982).
Acknowledgements
We thank G. van der Brugghen and D. van der Brugghen for the acquisition of the fossils; T. Sasaki and C. Sakata for assistance with fossil preparation; P. E. Ahlberg, C. Burrow, G. C. Young, J. Lu, Y. Zhu, J. Long, L. Knuefing, Y. Iba, Y. Nakajima and H. Higashiyama for discussions; C. Cupello, P. M. Brito, Y. Yabumoto and S. Isogai for the acquisition of the SRXµCT datasets for N. forsteri; and A. Limaye for developing Drishti and optimizing the software for biological visualization. This work was supported by JSPS KAKENHI grant numbers JP17K18354 (to T.H.) and JP17H06385 (to S.K.).
Author information
Authors and Affiliations
Contributions
T.H. and S.K. conceived the project. T.H. and M.M. conducted the conventional µCT analyses of preservational status. T.H., K.U. and M.H. conducted the SRXµCT scanning. T.H. and Y.H. segmented the CT data. T.H. and Y.H. conducted histological and phylogenetic analyses. T.H., Y.H. and S.K. wrote the paper. All authors discussed the data and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Russell Garwood, Philippe Janvier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
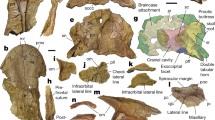
Extended Data Fig. 1 Cranial skeleton of P. gunni, NSMPV 24678.
a, Position of the cranial skeleton embedded intact within the matrix. b, Dorsal view. c, Ventral view. arp, arcual plate; aup1, autopalatinum 1; aup2, autopalatinum 2; bspp, basipterygoid process; epp, epipterygoid; hym, hyomandibula; ltc, lateral commissure; mc, Meckel's cartilage; mtp, metapterygoid; nac, nasal capsule; nas, nasal septum; ocpa, occipital arches (basioccipital portion of the neurocranium); prop, prootic process; qua, quadrate; vt1–7, vertebral segments 1–7. Scale bar, 1 mm.
Extended Data Fig. 2 Volume visualizations of P. gunni, NSMPV 24679, showing the distribution of cell lacunae within the skeletal tissues.
a–l, Serial transverse sections in ventral view, from ventral (a) to dorsal (l). Scale bar, 1 mm.
Extended Data Fig. 3 Phylogenetic position of Palaeospondylus.
a, Undated Bayesian analysis using MrBayes18. 50% majority-rule consensus of 27,777 trees (mean log-likelohood of 1,761.99). Numbers at nodes refer to posterior probabilities. b, Parsimony analysis using PAUP*19. Strict consensus of all 792 most-parsimonious trees (448 steps). Numbers at nodes refer to bootstrap percentages, based on 200 replicates. Taxonomic names in green, brown, blue, and reds indicate the dipnomorphs, rhizodonts, 'elpistostegalians', and limb-bearing tetrapods, respectively.
Supplementary information
Supplementary Information
This file contains details regarding the historical background, a detailed description of Palaeospondylus chondrocranium and the phylogenetic analysis, Supplementary Figs. 1–9 and references.
Supplementary Data 1
MrBayes executable file with a conservative interpretation regarding character 108 (Extended Data Fig. 3a).
Supplementary Data 2
PAUP* executable file with a conservative interpretation regarding character 108 (Extended Data Fig. 3b).
Supplementary Data 3
MrBayes executable file with an alternative interpretation regarding character 108 (Supplementary Fig. 9a).
Supplementary Data 4
PAUP* executable file with an alternative interpretation regarding character 108 (Supplementary Fig. 9b).
Supplementary Video 1
Cranial skeleton of P. gunni, NSMPV 24679.
Supplementary Video 2
Neurocranium of P. gunni, NSMPV 24679.
Supplementary Video 3
Volume visualizations of P. gunni, NSMPV 24679, showing the distribution of cell lacunae within the skeletal tissues, in dorsal view.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hirasawa, T., Hu, Y., Uesugi, K. et al. Morphology of Palaeospondylus shows affinity to tetrapod ancestors. Nature 606, 109–112 (2022). https://doi.org/10.1038/s41586-022-04781-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04781-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.