Abstract
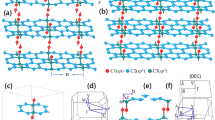
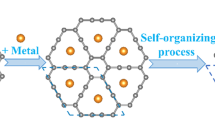
Two-dimensional (2D) carbon materials, such as graphene, have attracted particular attention owing to the exceptional carrier transport characteristics that arise from the unique π-electron system in their conjugated carbon network structure1,2,3,4. To complement zero-bandgap graphene, material scientists have devoted considerable effort to identifying 2D carbon materials5,6,7,8. However, it is a challenge to prepare large-sized single-crystal 2D carbon materials with moderate bandgaps5,9. Here we prepare a single-crystal 2D carbon material, namely monolayer quasi-hexagonal-phase fullerene (C60), with a large size via an interlayer bonding cleavage strategy. In this monolayer polymeric C60, cluster cages of C60 are covalently bonded with each other in a plane, forming a regular topology that is distinct from that in conventional 2D materials. Monolayer polymeric C60 exhibits high crystallinity and good thermodynamic stability, and the electronic band structure measurement reveals a transport bandgap of about 1.6 electronvolts. Furthermore, an asymmetric lattice structure endows monolayer polymeric C60 with notable in-plane anisotropic properties, including anisotropic phonon modes and conductivity. This 2D carbon material with a moderate bandgap and unique topological structure offers an interesting platform for potential application in 2D electronic devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this work are available within the paper and its Extended Data. Source data are provided with this paper.
References
Geim, A. K. & Novoselov, K. S. The rise of graphene. Nat. Mater. 6, 183–191 (2007).
Wang, Q. H., Kalantar-Zadeh, K., Kis, A., Coleman, J. N. & Strano, M. S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7, 699–712 (2012).
Li, L. et al. Black phosphorus field-effect transistors. Nat. Nanotechnol. 9, 372–377 (2014).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Fan, Q. et al. Biphenylene network: a nonbenzenoid carbon allotrope. Science 372, 852–856 (2021).
Kolmer, M. et al. Rational synthesis of atomically precise graphene nanoribbons directly on metal oxide surfaces. Science 369, 571–575 (2020).
Yu, H., Xue, Y. & Li, Y. Graphdiyne and its assembly architectures: synthesis, functionalization, and applications. Adv. Mater. 31, e1803101 (2019).
Bakharev, P. V. et al. Chemically induced transformation of chemical vapour deposition grown bilayer graphene into fluorinated single-layer diamond. Nat. Nanotechnol. 15, 59–66 (2020).
Toh, C. T. et al. Synthesis and properties of free-standing monolayer amorphous carbon. Nature 577, 199–203 (2020).
Cui, X. et al. Rolling up transition metal dichalcogenide nanoscrolls via one drop of ethanol. Nat. Commun. 9, 1301 (2018).
Wan, J. et al. Ultra-thin solid electrolyte interphase evolution and wrinkling processes in molybdenum disulfide-based lithium-ion batteries. Nat. Commun. 10, 3265 (2019).
Hirsch, A. The era of carbon allotropes. Nat. Mater. 9, 868–871 (2010).
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 7, 845–854 (2008).
Cao, Y. et al. Unconventional superconductivity in magic-angle graphene superlattices. Nature 556, 43–50 (2018).
Zhai, H. J. et al. Observation of an all-boron fullerene. Nat. Chem. 6, 727–731 (2014).
Jena, P. & Sun, Q. Super atomic clusters: design rules and potential for building blocks of materials. Chem. Rev. 118, 5755–5870 (2018).
Blank, V. D. et al. High-pressure polymerized phases of C60. Carbon 36, 319–343 (1998).
Okada, S. & Saito, S. Electronic structure and energetics of pressure-induced two-dimensional C60 polymers. Phys. Rev. B 59, 1930–1936 (1999).
Xu, C. H. & Scuseria, G. E. Theoretical predictions for a two-dimensional rhombohedral phase of solid C60. Phys. Rev. Lett. 74, 274–277 (1995).
Makarova, T. L. et al. Magnetic carbon. Nature 413, 716–718 (2001).
Tanaka, M. & Yamanaka, S. Vapor-phase growth and structural characterization of single crystals of magnesium doped two-dimensional fullerene polymer Mg2C60. Cryst. Growth Des. 18, 3877–3882 (2018).
Pekker, S. et al. Single-crystalline (KC60)n: a conducting linear alkali fulleride polymer. Science 265, 1077–1078 (1994).
Porezag, D., Pederson, M. R., Frauenheim, T. & Kohler, T. Structure, stability, and vibrational properties of polymerized C60. Phys. Rev. B 52, 14963–14970 (1995).
Haddon, R. C. et al. Conducting films of C60 and C70 by alkali-metal doping. Nature 350, 320–322 (1991).
Wågberg, T. & Sundqvist, B. Raman study of the two-dimensional polymers Na4C60 and tetragonal C60. Phys. Rev. B 65, 155421 (2002).
Long, V. C. et al. Far-infrared vibrational properties of high-pressure high-temperature C60 polymers and the C60 dimer. Phys. Rev. B 61, 13191–13201 (2000).
Chen, Y. et al. Black arsenic: a layered semiconductor with extreme in-plane anisotropy. Adv. Mater. 30, e1800754 (2018).
Xia, F., Wang, H. & Jia, Y. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 5, 4458 (2014).
Acknowledgements
J.Z. acknowledges support from the National Key Research and Development Program of China 2017YF0204700, the Key Research Program of the Chinese Academy of Sciences, grant number XDPB13, and the National Natural Science Foundation (22175184, 22105207). We thank Y. Cheng for the assistance with FIB-SEM; X. Hao and T. Liang for the assistance with the single-crystal XRD data analysis; and Y. Zou for the assistance with the UPS and LEIPS analyses.
Author information
Authors and Affiliations
Contributions
J.Z. designed the research. L.H. performed the exfoliation approach and analysed the results. X.C., S.W., R.L., Y.L. and D.Z. assisted with the exfoliation approach and the analysis of the results. B.G. performed the electron microscopy analyses. J.Z. supervised the research. J.Z., L.H. and X.C. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Michael Gottfried, Peter Stephens and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Schematic illustration of C60 cage in qHP C60 and qTP C60.
a, In qHP C60, each C60 cage forms two [2+2] cycloadditon C-C bonds and four C-C single bonds with neighbouring C60 cages. Thus there are only 52 carbon atoms possess π states in each C60 cage. b, Differing from that in qHP C60, two [2+2] cycloadditon C-C bonds and two C-C single bonds are formed with neighbouring C60 cages in qTP C60, therefore 54 carbon atoms possess π states in each C60 cage.
Extended Data Fig. 2 Exfoliation mechanism exploration.
a, Magnesium salicylate is obtained from the reaction system by chromatographic separation. The peak at 297.02543 corresponds to [M-H]- signal of magnesium salicylate. b, XRD spectra of Mg intercalated qHP C60 before (red) and after (black) ion-exchange reaction. In Mg intercalated bulk qHP C60, interlayer distance is characterized by (200) peak in 2θ = 10.7°. After ion-exchange reaction, the (200) peak moves to lower 2θ position, which indicate the expanding of interlayer distance. The peak in 2θ = 8.8° (interlayer distance of 10 Å) is attributed to the intercalation of NBu4+ ions. As NBu4+ ions are flexible, the expanded (200) peaks in exfoliated polymeric C60 are broader than that in bulk crystals.
Extended Data Fig. 3 Statistical analysis of the lateral sizes and proportion of monolayer for exfoliated qHP C60 flakes.
a, Typical optical image of the exfoliated qHP C60 flakes on SiO2/Si substrate. Scale bar, 50 μm. b, The statistics of lateral sizes of monolayer qHP C60. c, Statistics of thickness of exfoliated qHP C60 flakes.
Extended Data Fig. 4 Optical bandgap of exfoliated qHP C60.
Tauc plot to determine the optical bandgap of qHP C60, with extrapolation of the linear region (red line) estimating of approximately 1.55 eV.
Extended Data Fig. 5 Monolayer qHP C60 transistors.
a, Transfer characteristics of monolayer qHP C60 transistors. Inset is a schematic of the device. b, Output characteristics of the device in a.
Extended Data Fig. 6 Environment stability (a, b) and thermal stability (c) of the exfoliated qHP C60.
a, b, Photograph of exfoliated qHP C60 nanosheets dispersed in NMP before (a) and after (b) standing in air for one month. The solution retained clear without sediment or aggregation. c, Raman spectra and optical images of exfoliated qHP C60 flakes before (red) and after (black) heating at 600 K for ten minutes. The Raman spectrum and optical image remain unchanged after heating, indicating that the polymeric C60 frameworks in exfoliated qHP C60 do not decompose at 600 K.
Extended Data Fig. 7 XPS spectra for bulk qHP C60 (upper) and monolayer qHP C60 (bottom).
For monolayer qHP C60, peak of Mg 1s is absent, and the appearing N 1s peak at 401 eV binding energy is typical for quaternary ammonium groups. The O 1s (532 eV) and O 1s (533 eV) peaks correspond to oxygen from surrounding environment and the SiO2 substrate, respectively. Meanwhile, absence of typical carboxyl group peak in O 1s (535 eV) indicates that there is no residual salicylate on monolayer flakes.
Extended Data Fig. 8 C 1s XPS spectra for monolayer qHP C60.
C 1s peak can be consistently deconvoluted into several components attributed to sp2-hybridized C-C (284.6 eV), sp3-hybridized C-C (284.9 eV), sp3-hybridized C-N (286.8 eV) with approximately 52:80:24 intensity ratios, in agreement with the C60/NBu4+ ratio of 1:6 in monolayer qHP flakes. Moreover, a series of π-π* shake-up satellite peaks of C60 are identified in 286.2eV, 288.3 eV, 289.4 eV and 290.6 eV.
Extended Data Fig. 9 Zeta potential distribution of monolayer qHP C60 solution before (black) and after (red) treatment with hydrogen peroxide.
Average zeta potential of monolayer qHP C60 solution is calculated as −40.2 mV, indicating the negative charge on monolayer flakes. After oxidization, negative charges are neutralized, and average zeta potential turns to −8.4 mV.
Extended Data Fig. 10 XPS spectra for 3% H2O2 treated monolayer qHP C60.
The absence of N 1s peak indicates the removal of NBu4+ cations on the monolayer after the treatment of H2O2. Besides, C-O bonds are not formed, because typical C-O peak at 286–287 eV is not found. The O 1s peak is attributed to the adsorbed oxygen from the surrounding environment.
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
Hou, L., Cui, X., Guan, B. et al. Synthesis of a monolayer fullerene network. Nature 606, 507–510 (2022). https://doi.org/10.1038/s41586-022-04771-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04771-5
This article is cited by
-
Proposing TODD-graphene as a novel porous 2D carbon allotrope designed for superior lithium-ion battery efficiency
Scientific Reports (2024)
-
Synthesis of inter-[60]fullerene conjugates with inherent chirality
Nature Communications (2024)
-
Promising sensors for pharmaceutical pollutant adsorption using Clar’s goblet-based 2D membranes
Scientific Reports (2024)
-
Masked alkynes for synthesis of threaded carbon chains
Nature Chemistry (2024)
-
Strain-restricted transfer of ferromagnetic electrodes for constructing reproducibly superior-quality spintronic devices
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.