Abstract
Cellular diversification is critical for specialized functions of the brain including learning and memory1. Single-cell RNA sequencing facilitates transcriptomic profiling of distinct major types of neuron2,3,4, but the divergence of transcriptomic profiles within a neuronal population and their link to function remain poorly understood. Here we isolate nuclei tagged5 in specific cell types followed by single-nucleus RNA sequencing to profile Purkinje neurons and map their responses to motor activity and learning. We find that two major subpopulations of Purkinje neurons, identified by expression of the genes Aldoc and Plcb4, bear distinct transcriptomic features. Plcb4+, but not Aldoc+, Purkinje neurons exhibit robust plasticity of gene expression in mice subjected to sensorimotor and learning experience. In vivo calcium imaging and optogenetic perturbation reveal that Plcb4+ Purkinje neurons have a crucial role in associative learning. Integrating single-nucleus RNA sequencing datasets with weighted gene co-expression network analysis uncovers a learning gene module that includes components of FGFR2 signalling in Plcb4+ Purkinje neurons. Knockout of Fgfr2 in Plcb4+ Purkinje neurons in mice using CRISPR disrupts motor learning. Our findings define how diversification of Purkinje neurons is linked to their responses in motor learning and provide a foundation for understanding their differential vulnerability to neurological disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Sample information, cerebellum cluster maker gene list, differentially expressed genes of Plcb4+ and Aldoc+ Purkinje neurons and module significant genes are provided in the Supplementary Information. All source data, including sequencing reads and single-cell expression matrices are available from the Gene Expression Omnibus (GEO) under accession code GSE153184.
Code availability
Code for preprocessing of calcium imaging or optogenetics and snRNA-seq bioinformatic analysis are available from the authors on request.
References
Yap, E. L. & Greenberg, M. E. Activity-regulated transcription: bridging the gap between neural activity and behavior. Neuron 100, 330–348 (2018).
Mayer, C. et al. Developmental diversification of cortical inhibitory interneurons. Nature 555, 457–462 (2018).
Kozareva, V. et al. A transcriptomic atlas of mouse cerebellar cortex comprehensively defines cell types. Nature 598, 214–219 (2021).
Hrvatin, S. et al. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat. Neurosci. 21, 120–129 (2018).
Mo, A. et al. Epigenomic signatures of neuronal diversity in the mammalian brain. Neuron 86, 1369–1384 (2015).
Yang, Y. et al. Chromatin remodeling inactivates activity genes and regulates neural coding. Science 353, 300–305 (2016).
De Zeeuw, C. I. Bidirectional learning in upbound and downbound microzones of the cerebellum. Nat. Rev. Neurosci. 22, 92–110 (2021).
Zhou, H. et al. Cerebellar modules operate at different frequencies. eLife 3, e02536 (2014).
Wadiche, J. I. & Jahr, C. E. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat. Neurosci. 8, 1329–1334 (2005).
Rodriques, S. G. et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019).
McConnell, M. J., Huang, Y. H., Datwani, A. & Shatz, C. J. H2-Kb and H2-Db regulate cerebellar long-term depression and limit motor learning. Proc. Natl Acad. Sci. USA 106, 6784–6789 (2009).
Yamada, T. et al. Sensory experience remodels genome architecture in neural circuit to drive motor learning. Nature 569, 708–713 (2019).
Dana, H. et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019).
El-Shamayleh, Y., Kojima, Y., Soetedjo, R. & Horwitz, G. D. Selective optogenetic control of Purkinje cells in monkey cerebellum. Neuron 95, 51–62.e54 (2017).
Sarna, J. R., Marzban, H., Watanabe, M. & Hawkes, R. Complementary stripes of phospholipase Cβ3 and Cβ4 expression by Purkinje cell subsets in the mouse cerebellum. J. Comp. Neurol. 496, 303–313 (2006).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008).
Thomas, G. M. & Huganir, R. L. MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 5, 173–183 (2004).
Spencer, J. A., Major, M. L. & Misra, R. P. Basic fibroblast growth factor activates serum response factor gene expression by multiple distinct signaling mechanisms. Mol. Cell. Biol. 19, 3977–3988 (1999).
Herrup, K. & Wilczynski, S. L. Cerebellar cell degeneration in the leaner mutant mouse. Neuroscience 7, 2185–2196 (1982).
Sarna, J. R. et al. Patterned Purkinje cell degeneration in mouse models of Niemann–Pick type C disease. J. Comp. Neurol. 456, 279–291 (2003).
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Zhu, S. et al. Genome-scale deletion screening of human long non-coding RNAs using a paired-guide RNA CRISPR–Cas9 library. Nat. Biotechnol. 34, 1279–1286 (2016).
Sun, H. et al. Development of a CRISPR–SaCas9 system for projection- and function-specific gene editing in the rat brain. Sci. Adv. 6, eaay6687 (2020).
Andermann, M. L., Kerlin, A. M., Roumis, D. K., Glickfeld, L. L. & Reid, R. C. Functional specialization of mouse higher visual cortical areas. Neuron 72, 1025–1039 (2011).
Sparta, D. R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 7, 12–23 (2011).
Deverett, B., Kislin, M., Tank, D. W. & Wang, S. S. Cerebellar disruption impairs working memory during evidence accumulation. Nat. Commun. 10, 3128 (2019).
Giovannucci, A. et al. CaImAn an open source tool for scalable calcium imaging data analysis. eLife 8, e38173 (2019).
Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: An online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Methods 291, 83–94 (2017).
Spiegel, I. et al. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 157, 1216–1229 (2014).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Mi, H., Muruganujan, A., Casagrande, J. T. & Thomas, P. D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 8, 1551–1566 (2013).
Acknowledgements
This work was supported by NIH grant NS041021 (to A.B.), National Key Research and Development Program of China grant 2018YFA0108000, 2019YFA0110300 (to X.Z.), National Natural Science Foundation of China grant 82025020 (to X.Z.), F32 MH120887 (to G.J.B.), and R01 NS045193 and R01 MH115750 (to S.W.), Children’s Discovery Institute of Washington University and St Louis Children’s Hospital (CDI-CORE-2015-505 and CDI-CORE-2019-813) and the Foundation for Barnes-Jewish Hospital (3770 and 4642). Behavior Core was supported by funds provided by the McDonnell Center for Systems Neuroscience at Washington University in St Louis.
Author information
Authors and Affiliations
Contributions
X.C. and A.B. designed the study and wrote the manuscript. X.C. performed experiments and analysed data, assisted by C.M.Y. and S.Z. G.J.B., M.K. and S.S.-H.W. performed calcium imaging and optogenetics experiments. Y.D., X.C., S.D., X.Z., G.Z. and H.G. performed sequencing analyses. All authors discussed the results and commented on or edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
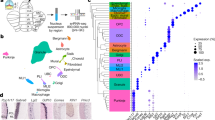
Extended Data Fig. 1 An INTACT-FACS-snRNA-seq system for high throughput transcriptomic analysis of cell types in adult mouse cerebellum.
a, Adult Pcp2-Cre/Sun1fl/wt mouse cerebellum was subjected to immunohistochemical analyses with antibodies labeling Calbindin (red), GFP (green) and the DNA dye bisbenzimide (Hoechst). INTACT labeling was restricted to Calbindin+ Purkinje neurons. Scale bar, 100 μm. Similar results were found in 4 animals for representative data. b, Schematic representation of the sagittal view of lobules across the central vermis and top view of the whole cerebellum. Parasagittal Zebrin II+ strips are labeled in light color and anatomically dissected anterior vermis lobules are boxed. c, Adult mouse cerebellum was subjected to INTACT gradient nuclei isolation followed by FACS purification. GFP positive or negative while DyeCycle Ruby labeled single nuclei were sorted and defined as qualified for future sequencing analysis. d, Nuclei from pre- and post-FACS were subjected to DNA dye bisbenzimide analysis under the confocal microscope. Preserved intact nuclei after FACS were observed. Scale bar, 10 μm. Similar results were found in 2 animals for representative data. e, f, Nuclei from pre- and post-FACS were subjected to qRT-PCR analyses, and the mRNA expressions of Pcp2 and Gabra6 were either enriched or de-enriched in post-FACS nuclei samples. Data are presented as mean ± s.e.m. ***p = 6.9e-6, 5.6e-6, by two-tailed unpaired t-test, n = 4 mice. g, h, i, Pre- and post-FACS nuclei from cerebellum of baseline mice or mice with rotarod activation were subjected to qRT-PCR analysis of the Fos, FosB and Npas4 expressions. Data are presented as mean ± s.e.m. ***p = 1.9e-5, 2.7e-7 for pre- and post-FACS Fos expression; ***p = 3.0e-4, 3.7e-4 for pre- and post-FACS FosB expression, **p = 0.003, 0.003 for pre- and post-FACS Npas4 expression, n = 4 mice, two-tailed unpaired t-test. j, 18,151 nuclei from anterior vermis lobules assigned into granule neurons, Purkinje neurons, interneurons, glial cells and oligodendrocytes based on unsupervised clustering and UMAP plot visualization. k, l, Nuclei of two biological replicates and sequencing batches from the whole cerebellum or anterior vermis lobules showed similar cell type clustering and high correlation of gene expression. m, Feature plot demonstrated the number of nCounts in each categorized nuclei isolated from the whole cerebellum or anterior vermis lobules. n, Feature plot demonstrated that the GFP transcripts almost exclusively enriched in Pcp2 featured Purkinje neurons. 98.1% GFP+ cells in the whole cerebellum and 95.9% in the anterior vermis lobules were clustered with the Purkinje neuron population. o, Heat map showing identified marker genes in each categorized cell types.
Extended Data Fig. 2 Aldoc+ and Plcb4+ Purkinje neurons show distinct molecular and cellular features.
a, 20,814 single nuclei of Purkinje neurons were subjected to principal component analysis. Heat map showed that the first principal component (60.9%) clustered Purkinje neurons into Plcb4+ and Aldoc+ populations. b, UMAP plot showing grouped Purkinje nuclei from the whole cerebellum or anterior vermis lobules into two primary categories, Aldoc+ and Plcb4+ Purkinje neurons. c, Nuclei of two biological replicates and sequencing batches from the whole cerebellum or anterior vermis lobules showed similar cell type clustering. d, Bar plot showing the percentages of Aldoc+ and Plcb4+ Purkinje neurons in the whole cerebellum and anterior vermis lobules. Data are presented as mean ± s.e.m. n = 2 in biologically independent snRNA-seq. 5870 Aldoc+ and 5675 Plcb4+ neurons in whole cerebellum; 3243 Aldoc+ and 6026 Plcb4+ neurons in anterior vermis lobules. e, The coronal sections through the anterior (up panel) or posterior (lower panel) cerebellar lobules were subjected to ISH analyses using fluorescence-labeled Aldoc (red) or Plcb4 (green) RNA probes. Scale bar, 800 μm. Similar results were found in 3 animals for representative data. f, Scatter plot depicting the expressions of DEGs in Plcb4+ and Aldoc+ Purkinje neurons in the anterior vermis lobules. Blue dots showing representative genes highly expressed in Plcb4+, while purple ones were those in Aldoc+ Purkinje neurons. g, Heat map showing the representative functional genes specifically expressed in Plcb4+ or Alodc+ Purkinje neurons isolated from the whole cerebellum or anterior vermis lobules. Expression values are presented as normalized Z-scores.
Extended Data Fig. 3 ISH validation of the expression patterns of newly identified cluster genes for Purkinje neurons in sagittal sections.
a, b, c, d, e, ISH analyses using fluorescence-labeled Aldoc or Plcb4 RNA probes (green) and probes for newly identified cluster genes (red) in cerebellar sagittal sections through the central vermis. Scale bars, 800 μm for the left panel and 200 μm for the right panel. Similar results were found in 3 animals for representative data.
Extended Data Fig. 4 ISH validation of the expression pattern of newly identified cluster genes for Purkinje neurons in coronal sections.
a, b, c, ISH analyses using fluorescence-labeled Aldoc or Plcb4 RNA probes (green) and probes for newly identified cluster genes (red) in cerebellar coronal sections. Scale bars, 800 μm for the left panel and 200 μm for the right panel. Similar results were found in 3 animals for representative data.
Extended Data Fig. 5 Plcb4+ Purkinje neurons undergo transcriptomic plasticity upon exposure to motor activity and learning.
a, Schematic representation of the training paradigms for activity and motor learning. b, Cerebellar motor learning paradigm was performed on Pcp2-cre/sun1fl/wt mice. Data are presented as mean ± s.e.m. n = 9 mice. c, Schematic representation of the delay tactile startle conditioning training paradigm for associative learning. CS, conditional stimuli with blue light; US, unconditional stimuli with a motorized toy. d, e, Cerebellar associative learning paradigm was performed on Pcp2-cre/sun1fl/wt mice. Data are presented as mean ± s.e.m. n = 11 mice. f, ElbowPlot showing the ordered standard deviations of principal components (PCs) for identification of significant dimension that begins to plateau. Dim 8 was chosen at the standard deviations of 2. g, Heat map showing the representative marker genes of each cluster of Plcb4+ Purkinje neurons isolated from the whole cerebellum or anterior vermis lobules. Expression values are presented as normalized Z-scores. h, 22,205 Plcb4+ Purkinje nuclei isolated from the whole cerebellum or anterior lobules under control, activity, motor learning or associative learning conditions were grouped into 10 clusters based on marker gene expression with cluster resolution of 1.0. i, Bar plot showing the percentages of all 10 clusters with cluster resolution at 1.0 under different conditions described in h. j, 22,205 Plcb4+ Purkinje nuclei isolated from the whole cerebellum or anterior lobules under control, activity, motor learning or associative learning conditions were grouped into 4 clusters based on marker gene expression with cluster resolution of 0.2. k, Monocle analysis showing C3/C4 and C0/C2 were clearly demarcated into two major paths: C4 derived from C3 and C2 derived from C0. l, 103 upregulated genes along the pseudo-time course referring to activity/learning-linked genes in C2/C4 and 26 downregulated genes linked with C0/C3 were identified, respectively. m, Dynamic expression changes of C2/C4 representative gene, Ube3a, and C0/C3 representative gene, Nup93, along the pseudo-time course.
Extended Data Fig. 6 Aldoc+ Purkinje neurons show no obvious transcriptomic plasticity upon exposure to motor activity and learning.
a, ElbowPlot showing the ordered standard deviations of principal components (PCs) for identification of significant dimension that begins to plateau. Dim 8 was chosen at the standard deviations of 2. b, 19,424 Aldoc+ Purkinje nuclei isolated from the whole cerebellum or anterior vermis lobules under control, activity, motor learning or associative learning conditions were grouped into 4 clusters based on marker gene expression with cluster resolution of 0.2. c, 19,424 Aldoc+ Purkinje nuclei isolated from the whole cerebellum or anterior vermis lobules under control, activity, motor learning or associative learning conditions were grouped into 12 clusters based on marker gene expression with cluster resolution of 1.0. d, e, UMAP plot showing the grouped cluster landscape of Aldoc+ nuclei of different training paradigms under baseline, activity, learning or learned conditions. f, Bar plot showing the percentages of all 12 clusters with cluster resolution of 1.0 under different conditions described in c. g, Heat map showing the representative marker genes of each cluster (resolution of 0.5) of Aldoc+ Purkinje neurons isolated from the whole cerebellum or anterior vermis lobules. Expression values are presented as normalized Z-scores. h, Fos expression in the clusters of Plcb4+ and Aldoc+ Purkinje neurons at baseline or learning/learned condition.
Extended Data Fig. 7 Plcb4+, but not Aldoc+ Purkinje neurons undergo transcriptomic plasticity upon exposure to motor activity and learning.
a, Two biological replicates of combined Aldoc+ and Plcb4+ Purkinje nuclei were grouped into 12 clusters based on marker gene expression with cluster resolution of 1.0 (Dim 12). b, Projection of 6 clusters of Plcb4+ neurons in Fig. 2a and 6 clusters of Aldoc+ neurons in Fig. 2g to the combined dataset. Cluster 9 and cluster 11 were respectively categorized with C2 and C4, those two Plcb4+ clusters enriched in activity and learning experiences. c, Split visualization of the 12 clusters of Aldoc+ and Plcb4+ Purkinje neurons isolated from the whole cerebellum or anterior vermis lobules from two replicates under activity, motor learning or associative learning conditions. d, Bar plot showing the percentages of all 12 clusters of Aldoc+ and Plcb4+ Purkinje neurons isolated from the whole cerebellum or anterior vermis lobules as demonstrated in c. e, Fos expression in the clusters of combined Plcb4+ and Aldoc+ Purkinje neurons at baseline or learning/learned condition.
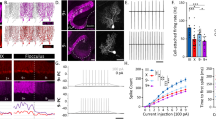
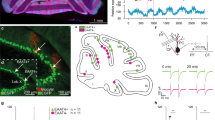
Extended Data Fig. 8 Plcb4+ Purkinje neuron activity is required for associative learning.
a, AAV-Plcb4-tdTomato labels Plcb4+ Purkinje neurons transduced by Purkinje neuron-specific GCaMP (AAV-L7-cre + rAAV-CAG-FLEx-jGCaMP7f). Scale bar, 50 μm. Similar results were found in 2 animals for representative data. b, AAV-Plcb4-ChR2-mCherry representative histology. Scale bars, 100 μm for the top panel and 50 μm for the lower panels. Similar results were found in 2 animals for representative data. c, Quantification analysis of mCherry intensities in Plcb4+ and Aldoc+ Purkinje neurons as in b. Data are presented as standard box and whisker plots. ***p = 1.2e-9, by two-tailed unpaired t-test. n = 301 Plcb4+ and 113 Aldoc+ Purkinje neurons derived from 3 mice. d, (Left panel) Schematic representation of the whole cerebellum, anterior cerebellum and light-spatially-targeted ADCV. (Right panel) Comparison of the ratio of Plcb4+ to all Purkinje neurons extracted in whole cerebellum (0.49 ± 0.005, n = 2 biologically independent experiments), ADCV (0.65 ± 0.02, n = 2 biologically independent experiments) (as shown in Extended Data figure 2d), excited by spatially directed optogenetic light (PcP2-Ai27D targeted light, 0.89 ± 0.04, 12 slices derive from 4 mice) and Plcb4- specific optogenetic experiments (Plcb4-ChR2-mCherry, 0.95 ± 0.01, cell counts from 3 mice). Data are presented as mean ± s.e.m. e, Fraction of trials with a CR during sessions with light-on or light-off optostimulation for animals transduced by Plcb4-ChR2 with L7-cre (n = 7 mice) or L7-Cre alone (controls, n = 4 mice). As evidenced by CR performance during the first five training sessions, daily optostimulation of Plcb4+ Purkinje neurons in lobules IV/V impairs associative learning in mice. During optostimulation light off sessions, Plcb4-ChR2 mice show gradual increase in CR performance similar to L7-control mice. Data are presented as mean ± s.e.m. p = 0.03, 0.005, 0.01 for days 4–6, by two-tailed unpaired t-test. f, Average UR on session 1 (upper left panel). Unlike Plcb4-ChR2 mice, control mice learned to make well-timed CRs by session 5 despite equivalent exposure to light-on optostimulation (lower left panel). Both groups exhibit well-timed CRs with light-off optostimulation by session 10 (lower right panel). g, The correlation of ChR2 expression with CR success rate at training section 5 and 6.
Extended Data Fig. 9 Motor activity- and learning-associated gene modules in Purkinje neurons.
a, Weighted gene co-expression network analysis (WGCNA) dendrogram indicating clustering of different gene modules in Plcb4+ and Aldoc+ nuclei upon activity, motor learning or associative learning training. b, FeaturePlot showing the average expression of the activation (brown/black) and learning (blue) module genes in clusters of Plcb4+ and Aldoc+ Purkinje neurons. c, Gene ontology (GO) and Pathway analyses of the activity (brown/black) modules. d, Hub-gene network analysis of the activity (brown/black) modules. e, Enrichment of the learning genes in Plcb4+ (left panel), Plcb4+ and Aldoc+ (right up panel), or Aldoc+ (right lower panel) Purkinje neurons under motor learning and associative learning paradigms. f, GO and Pathway analyses of Plcb4+, Plcb4+ and Aldoc+, or Aldoc+ Purkinje neurons-enriched learning genes. g, Enrichment of FGFR2 signaling regulated downstream transcription factor binding motifs, serum response element (SRE) and E-Twenty Six (ETS), in the learning (blue), activity (brown/black) and other module gene sets. Enrichment of SRE and ETS in random genome DNA sequences is used as control.
Extended Data Fig. 10 Specificity and efficiency of Plcb4-SaCas9 expression and Fgfr2 gRNA targeting.
a, Schematic representation of the arrangement of mouse Fgfr2 exons and the two targeting gRNAs. b, Schematic representation of the AAV vectors of Plcb4-SaCas9, U6-Scramble gRNA and U6-Fgfr2 gRNAs. c, Sequencing analyses showing an example of insertion/deletions (indels) mediated by SaCas9/Fgfr2 gRNA1 and gRNA2 in mouse Fgfr2 genomic loci after co-expression of SaCas9 and Fgfr2 gRNAs in mouse embryonic fibroblasts. d, Predicted off-target sites of Fgfr2 gRNAs. e, Genomic DNA sequences surrounding the targeting or off-targeting loci were amplified by PCR and ligated to T vector. After transformation, colonies of each group were sequenced and the percentage of colonies showing indels were calculated. f, Sequencing analyses showing examples of indels in Fgfr2 genomic loci of INTACT-enriched Purkinje neuron nuclei after AAV-mediated expression of SaCas9 and Fgfr2 gRNAs. g, Percentage of indels introduced in mouse Fgfr2 genomic loci of Purkinje neurons after AAV-mediated expression of SaCas9 and Fgfr2 gRNAs. h, Representative immunofluorescence images of Fgfr2 and Calbindin or Plcb4 in lobules IV/V in cerebellar sagittal sections of scramble or Fgfr2-KO mice. Scale bar, 100 μm. i, Split UMAP plot visualization of granule neurons, Purkinje neurons, interneurons, glial cells and oligodendrocytes from anterior vermis lobules of scramble and Fgfr2-KO samples under baseline and learned conditions. j, Visualization of molecular signatures of each population by their percentage and average gene expressions. k, Heat map of INTACT-FACS-snRNA-seq data from the anterior vermis lobules of Fgfr2-KO or control mice, presented as normalized Z-scores. l, Scatter plot showing the differential expression indices for each DEG in Aldoc+ or Plcb4+ Purkinje neurons upon Fgfr2-KO in Plcb4+ Purkinje neurons. m, Brown/black module genes were not reduced upon Fgfr2-KO in Plcb4+ cells. p = 1.08e-07 for scramble and p = 2.2e-16 for Fgfr2-KO, two-sided unpaired t-test. n = 2 in biologically independent snRNA-seq. 977 cells, 637 cells in scramble baseline and learned samples. 974 cells, 1514 cells in Fgfr2-KO baseline and learned samples.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1–6 and the full description for Supplementary Video 1.
Supplementary Video 1
Delay tactile startle conditioning learning.
Rights and permissions
About this article
Cite this article
Chen, X., Du, Y., Broussard, G.J. et al. Transcriptomic mapping uncovers Purkinje neuron plasticity driving learning. Nature 605, 722–727 (2022). https://doi.org/10.1038/s41586-022-04711-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04711-3
This article is cited by
-
Revisiting the development of cerebellar inhibitory interneurons in the light of single-cell genetic analyses
Histochemistry and Cell Biology (2024)
-
Neuronal types in the mouse amygdala and their transcriptional response to fear conditioning
Nature Neuroscience (2023)
-
Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy
Nature (2023)
-
Postsynaptic plasticity of Purkinje cells in mice is determined by molecular identity
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.