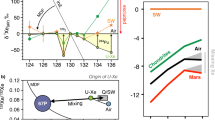
Abstract
Our understanding of atmosphere formation essentially relies on noble gases and their isotopes, with xenon (Xe) being a key tracer of the early planetary stages. A long-standing issue, however, is the origin of atmospheric depletion in Xe1 and its light isotopes for the Earth2 and Mars3. Here we report that feldspar and olivine samples confined at high pressures and high temperature with diluted Xe and krypton (Kr) in air or nitrogen are enriched in heavy Xe isotopes by +0.8 to +2.3‰ per amu, and strongly enriched in Xe over Kr. The upper +2.3‰ per amu value is a minimum because quantitative trapping of unreacted Xe, either in bubbles or adsorbed on the samples, is likely. In light of these results, we propose a scenario solving the missing Xe problem that involves multiple magma ocean stage events at the proto-planetary stage, combined with atmospheric loss. Each of these events results in trapping of Xe at depth and preferential retention of its heavy isotopes. In the case of the Earth, the heavy Xe fraction was later added to the secondary CI chondritic atmosphere through continental erosion and/or recycling of a Hadean felsic crust.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information file, and are available on the Zenodo repository (https://doi.org/10.5281/zenodo.6076901). Source data are provided with this paper.
References
Anders, E. & Owen, T. Mars and Earth: origin and abundance of volatiles. Science 198, 453–465 (1977).
Krummenacher, D., Merrihue, C. M., Pepin, R. O. & Reynolds, J. H. Meteoritic krypton and barium versus the general isotopic anomalies in xenon. Geochim. Cosmochim. Acta 26, 231–249 (1962).
Swindle, T. D., Caffee, M. W. & Hohenberg, C. M. Xenon and other noble gases in shergottites. Geochim. Cosmochim. Acta 50, 1001–1015 (1986).
Ozima, M. & Podosek, F. A. Formation age of Earth from 129I/127I and 244Pu/238U systematics and the missing Xe. J. Geophys. Res. 104, 25493–25499 (1999).
Avice, G., Marty, B. & Burgess, R. The origin and degassing history of the Earth’s atmosphere revealed by Archean xenon. Nat. Commun. 8, 15455 (2017).
Dauphas, N. & Morbidelli, A. in Geochemical and Planetary Dynamical Views on the Origin of Earth’s Atmosphere and Oceans (eds Holland, H. D. & Turekian, K. K.) 115–234 (Elsevier, 2014).
Pepin, R. O. On the origin and early evolution of terrestrial planet atmospheres and meteoritic volatiles. Icarus 92, 2–79 (1991).
Hébrard, E. & Marty, B. Coupled noble gas-hydrocarbon evolution of the early Earth atmosphere upon solar UV irradiation. Earth Planet. Sci. Lett. 385, 40–48 (2014).
Zahnle, K. J., Gaseca, M. & Catling, D. C. Strange messenger: a new history of hydrogen on Earth, as told by xenon. Geochim. Cosmochim. Acta 244, 56–85 (2019).
Dauphas, N. The dual origin of the terrestrial atmosphere. Icarus 165, 326–333 (2003).
Bekaert, D. V., Broadley, M. W. & Marty, B. The origin and fate of volatile elements on Earth revisited in light of noble gas data obtained from comet 67P/Churyumov–Gerasimenko. Sci. Rep. 10, 5796 (2020).
Marty, B. et al. Xenon isotopes in 67P/Churyumov–Gerasimenko show that comets contributed to Earth’s atmosphere. Science 356, 1069–1072 (2017).
Piani, L. et al. Earth’s water may have been inherited from material similar to enstatite chondrite meteorites. Science 50, 1110–1113 (2020).
Javoy, M. et al. The chemical composition of the Earth: enstatite chondrite models. Earth Planet. Sci. Lett. 293, 259–268 (2010).
Boyet, M. et al. Enstatite chondrites EL3 as building blocks for the Earth: the debate over the 146Sm–142Nd systematics. Earth Planet. Sci. Lett. 214, 427–442 (2018).
Sanloup, C. Noble gas reactivity in planetary interiors. Front. Phys. 8, 157 (2020).
Dewaele, A. et al. Synthesis and stability of xenon oxides Xe2O5 and Xe3O2 under pressure. Nat. Chem. 8, 784–790 (2016).
Stavrou, E. et al. Synthesis of xenon and iron-nickel intermetallic compounds at Earth’s core thermodynamic conditions. Phys. Rev. Lett. 120, 096001 (2018).
Crépisson, C., Blanchard, M., Lazzeri, M., Balan, E. & Sanloup, C. New constraints on Xe incorporation mechanisms in olivine from first-principles calculations. Geochim. Cosmochim. Acta 222, 146–155 (2018).
Probert, M. I. J. An ab initio study of xenon retention in α-quartz. J. Phys. Condens. Matter 22, 025501 (2010).
Crépisson, C. et al. The Xe-SiO2 system at moderate pressure and high temperature. Geochem. Geophys. Geosyst. 20, 992–1003 (2019).
Shcheka, S. S. & Keppler, H. The origin of the terrestrial noble-gas signature. Nature 490, 531–535 (2012).
Parai, R. & Mukhopadhyay, S. Xenon isotopic constraints on the history of volatile recycling into the mantle. Geochim. Cosmochim. Acta 560, 223–227 (2018).
Krantz, J. A., Parman, S. W. & Kelley, S. P. Recycling of heavy noble gases by subduction of serpentinite. Earth Planet. Sci. Lett. 521, 120–127 (2019).
Holland, G. & Ballentine, C. J. Seawater subduction controls the heavy noble gas composition of the mantle. Nature 441, 186–191 (2006).
Moreira, M., Kunz, J. & Allègre, C. Rare gas systematics in popping rock: isotopic and elemental compositions in the upper mantle. Science 279, 1178–1181 (1998).
Hennecke, E. W. & Manuel, O. K. Noble gases in Hawaiian xenolith. Nature 257, 778–780 (1975).
Poreda, R. J. & Farley, K. A. Rare gases in Samoan xenoliths. Earth Planet. Sci. Lett. 113, 129–144 (1992).
Czuppon, G., Matsumoto, T., Handler, M. R. & Matsuda, J.-I. Noble gases in spinel peridotite xenoliths from Mt Quincan, North Queensland, Australia: undisturbed MORB-type noble gases in the subcontinental lithospheric mantle. Chem. Geol. 266, 19–28 (2009).
Kuroda, P. K., Sherrill, R. D. & Jackson, K. C. Abundances and isotopic compositions of rare gases in granites. Geochem. J. 11, 75–90 (1977).
Palma, R. L., Rao, M. N., Rowe, M. W. & Koeberl, C. Krypton and xenon fractionation in North American tektites. Meteor. Planet. Sci. 32, 9–14 (1997).
Bekaert, D. V., Avice, G., Marty, B. & Henderson, B. Stepwise heating of lunar anorthosites 60025, 60215, 65315 possibly reveals an indigenous noble gas component on the Moon. Geochim. Cosmochim. Acta 218, 114–1315 (2017).
Drescher, J., Kirsten, T. & Schäfer, K. The rare gas inventory of the continental crust, recovered by the KTB Continental Deep Drilling project. Earth Plan. Sci. Lett. 154, 247–263 (1998).
Elkins-Tanton, L. T., Burgess, S. & Yin, Q.-Z. The lunar magma ocean: reconciling the solidification process with lunar petrology and geochronology. Earth Planet. Sci. Lett. 304, 326–336 (2011).
Frossard, P., Boyet, M., Bouvier, A., Hammouda, T. & Monteux, J. Evidence for anorthositic crust formed on an inner solar system planetesimal. Geochem. Persp. Lett. 11, 28–32 (2019).
Bouvier, L. C. et al. Evidence for extremely rapid magma ocean crystallization and crust formation on Mars. Nature 558, 586–589 (2018).
Caro, G., Bourdon, B., Birck, J.-L. & Moorbath, S. High-precision 142Nd/144Nd measurements in terrestrial rocks: constraints on the early differentiation of the Earth’s mantle. Geochim.Cosmochim. Acta 70, 164–191 (2006).
Harrison, T. M., Schmitt, A. K., McCulloch, M. T. & Lovera, O. M. Early (≥4.5 Ga) formation of terrestrial crust: Lu–Hf, δ18O, and Ti thermometry results for Hadean zircons. Earth Planet. Sci. Lett. 268, 476–486 (2008).
Erkaev, N. V. et al. Escape of the martian protoatmosphere and initial water inventory. Planet. Space Sci. 98, 106–119 (2014).
Tucker, J. M. & Mukhopadhyay, S. Evidence for multiple magma ocean outgassing and atmospheric loss episodes from mantle noble gases. Earth Planet. Sci. Lett. 393, 254–265 (2014).
Jambon, A., Weber, H. & Braun, O. Solubility of He, Ne, Ar, Kr and Xe in a basalt melt in the range 1250–1600 °C. Geochemical implications. Geochim. Cosmochim. Acta 50, 401–408 (1986).
Guillot, B. & Sator, N. Noble gases in high-pressure silicate liquids: a computer simulation study. Geochim. Cosmochim. Acta 80, 51–69 (2012).
Brož, M., Chrenko, O., Nesvorný, D. & Dauphas, N. Early terrestrial planet formation by torque-driven convergent migration of planetary embryos. Nat. Astron. 5, 898–902 (2021).
Schlichting, H. E. & Mukhopadhyay, S. Atmosphere impact losses. Space Sci. Rev. 214, 34 (2018).
Harper, C. L. Evidence for 92gNb in the early solar system and evaluation of a new p-process cosmochronometer from 92gNb/92Mo. Astrophys. J. 466, 437–456 (1996).
Jaupart, E., Charnoz, S. & Moreira, M. Primordial atmosphere incorporation in planetary embryos and the origin of neon in terrestrial planets. Icarus 293, 199–205 (2017).
Crépisson, C. et al. Kr environment in feldspathic glass and melt: a high pressure, high temperature X-ray absorption study. Chem. Geol. 493, 525–531 (2018).
Kohara, S. et al. Relationship between topological order and glass forming ability in densely packed enstatite and forsterite composition glasses. Proc. Natl Acad. Sci. USA 108, 14780–14785 (2011).
Holland, G., Cassidy, M. & Ballentine, C. J. Meteorite Kr in Earth’s mantle suggests a late accretionary source for the atmosphere. Science 326, 1522–1525 (2009).
Heber, V. S., Brooker, R. A., Kelley, S. P. & Wood, B. J. Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochim. Cosmochim. Acta 71, 1041–1061 (2007).
Sanloup, C., Schmidt, B. C., Gudfinnsson, G., Dewaele, A. & Mezouar, M. Xenon and argon: a contrasting behavior in olivine at depth. Geochim. Cosmochim. Acta 75, 6271–6284 (2011).
Péron, S. & Moreira, M. Onset of volatile recycling into the mantle determined by xenon anomalies. Geochem. Persp. Lett. 9, 21–25 (2018).
Tolstikhin, I. N. & O’nions, R. K. The Earth’s missing xenon: a combination of early degassing and of rare gas loss from the atmosphere. Chem. Geol. 115, 1–6 (1994).
Yokochi, R. & Marty, B. Geochemical constraints on mantle dynamics in the Hadean. Earth Planet. Sci. Lett. 238, 17–30 (2005).
Sano, Y., Marty, B. & Burnard, P. in Noble Gases in the Atmosphere (ed. Burnard, P.) 17–31 (Springer-Verlag, 2013).
Crépisson, C. ‘Missing Xenon’: Experimental and Theoretical Study of Xe Storage in Crustal and Upper Mantle Minerals. Ph.D. thesis, Sorbonne Univ. (2018).
Prouteau, G., Scaillet, B., Pichavant, M. & Maury, R. Evidence for mantle metasomatism by hydrous silicic melts derived from subducted oceanic crust. Nature 410, 197–200 (2001).
Boettcher, S. L., Guo, Q. & Montana, A. A simple device for loading gases in high-pressure experiments. Am. Mineral. 74, 1383–1384 (1989).
Horlait, D. et al. A new thermo-desorption laser-heating setup for studying noble gases diffusion and release from materials at high temperatures. Rev. Sci. Instr. 92, 124102 (2021).
Bevington, P. R. & Robinson, D. K. Data Reduction and Error Analysis for Physical Sciences 3rd edn (McGraw-Hill, 2003).
Acknowledgements
The research leading to these results was funded by the French CNRS PRIME80 and CNRS MITI Défi ISOTOP programmes. We acknowledge B. Lavielle and D. Bekaert for insightful discussions, R. Faure and B.A. Thomas for their technical assistance on MS measurements and T. Chematinov for his useful and appreciated participation in carrying out the step heating experiments. SEM measurements were done at the FIB and SEM facility at IMPMC, supported by Région Ile de France grant SESAME 2006 N°I-07-593/R, and by the French National Research Agency (ANR) grant no. ANR-07-BLAN-0124-01. We acknowledge G. Prouteau for providing the San Carlos olivine samples, and the mineralogical collection at Sorbonne Université for providing the feldspar samples.
Author information
Authors and Affiliations
Contributions
C.S. and D.H. devised the project, I.R. and C.S. carried out the high P-T experiments, I.R., D.H. and E.G. carried out analyses on the noble gas spectrometer. C.S. wrote the paper with input from I.R. and D.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Ray Burgess and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer files are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1
a, Xe fractionation as a function of the time spent between the synthesis and the mass spectrometry analysis, and b, Xe fractionation as a function of the sample mass. Data points for samples characterized, as expected, by an absence of detectable fractionation (runs at 1,400 °C or with 100% Xe loading gas) were removed from this Figure for the sake of clarity. Vertical error bars represent the SE for isotopic fractionation calculation
Extended Data Fig. 2 Summary of measured δn values in experiments realized with laser heating increasing steps.
Instead of analysing all Xe released after laser melting of the fragments, the tuneable heating laser was first set at lower powers (indicated as the power voltage V applied to the laser source). Each heating plateau was kept for a few minutes and the released Xe were analysed by mass spectrometry using the same general protocol described in the Methods section. A handful attempts were made on olivine and sanidine 1 samples. They all point toward Xe predominantly exiting the material at the fusion point (or close to). Only two experiments with sanidine, S1–11d with 1%Xe gas and S1–19b with 100%Xe gas, led to the successful measurement for all of the heating steps of both δn and [Xe] values, as reported in the present Figure. Each point area is proportional to the Xe content ([Xe]) extracted at the heating plateau. The stars are δn values combining all δn measured and weighted by each [Xe], in other words the δn we would had measured if the sample fragment had been directly melted. For S1–11d (1% Xe gas), we observe variations of δn by a roughly 2-fold factor along the heating ramp. For this same sample, an unfractionated component was evidenced for the lowest laser voltage, but represent a marginal part (5.3% of the total released Xe). Since for similar heating power, ~45% of the total Xe of S1–19b (100% Xe gas) was released, this low T release is possibly associated to Xe trapped as bubbles. At the highest T, i.e. at the sample melting point, for S1–19b and S1–11d respective 0.54 ±0.12 ‰/amu and 0.61 ±0.11 ‰/amu fractionations were measured, which points towards a Xe component with some fractionated Xe, but still with an unfractionated component lowering the overall measured δn. This indirectly confirms that Xe chemical incorporation and the associated isotopic fractionation occurs in all samples prepared at T ≤ 1,100 °C; δn close to zero for samples prepared with 100% Xe gas being only due to a disruptive phenomenon whose extent is proportional to Xe partial pressure: oversaturation of the mineral (bubble formation, as seen in Extended Data Fig. 3). Detailed data used to construct this Figure are found in the results Tables for S111d and S1–19b given in Supplementary information file, while synthesis conditions are found in Extended Data Table 1
Extended Data Fig. 3 SEM images in AsB mode.
a and b, Feldspar sanidine 1 loaded with 1 mol% Xe and 1 mol% Kr enriched air (identical to syntheses S1–13 and S1–14). Round dark area in b are synthesis gas bubbles revealed and opened by polishing. c, a sanidine 1 loaded with 100 mol% Xe (identical to syntheses S1–17, S1–18 and S1–19). Brighter areas in c are Xe bubbles, found in oversaturated samples, i.e. loaded with 100 mol% Xe gas.
Extended Data Fig. 4 Non-radiogenic Xe data reveal the possibility of Archean atmosphere contribution to mantle Xe within the present scenario of an early fractionated silicate Earth.
Xe measured in deep crustal fluids49 (black and grey circles), MORB popping rock52 (brown circle), air55, Archean atmosphere as trapped in crustal samples (dark blue and black squares); and primordial components (green and maroon circles). All data are shown with associated SE. Alternatively, the deep fluids and MORB popping rock enrichment in Xe light isotopes compared to air could be explained by input from a slightly less fractionated lower mantle resulting from the last magma ocean stage having affected only the upper mantle.
Supplementary information
Supplementary Data
Detailed mass spectrometry results.
Rights and permissions
About this article
Cite this article
Rzeplinski, I., Sanloup, C., Gilabert, E. et al. Hadean isotopic fractionation of xenon retained in deep silicates. Nature 606, 713–717 (2022). https://doi.org/10.1038/s41586-022-04710-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04710-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.