Abstract
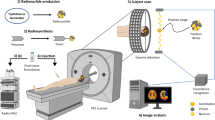
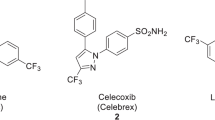
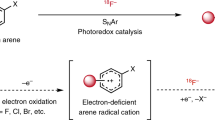
The advent of total-body positron emission tomography (PET) has vastly broadened the range of research and clinical applications of this powerful molecular imaging technology1. Such possibilities have accelerated progress in fluorine-18 (18F) radiochemistry with numerous methods available to 18F-label (hetero)arenes and alkanes2. However, access to 18F-difluoromethylated molecules in high molar activity is mostly an unsolved problem, despite the indispensability of the difluoromethyl group for pharmaceutical drug discovery3. Here we report a general solution by introducing carbene chemistry to the field of nuclear imaging with a [18F]difluorocarbene reagent capable of a myriad of 18F-difluoromethylation processes. In contrast to the tens of known difluorocarbene reagents, this 18F-reagent is carefully designed for facile accessibility, high molar activity and versatility. The issue of molar activity is solved using an assay examining the likelihood of isotopic dilution on variation of the electronics of the difluorocarbene precursor. Versatility is demonstrated with multiple [18F]difluorocarbene-based reactions including O–H, S–H and N–H insertions, and cross-couplings that harness the reactivity of ubiquitous functional groups such as (thio)phenols, N-heteroarenes and aryl boronic acids that are easy to install. The impact is illustrated with the labelling of highly complex and functionalized biologically relevant molecules and radiotracers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Materials and methods, optimization studies, experimental procedures, mechanistic studies, 1H NMR, 13C NMR and 19F NMR spectra, and high-resolution mass spectrometry, infrared and HPLC data are available in the Supplementary Information.
References
Reardon, S. Whole-body PET scanner produces 3D images in seconds. Nature 570, 285–287 (2019).
Ajenjo, J., Destro, G., Cornelissen, B. & Gouverneur, V. Closing the gap between 19F and 18F chemistry. EJNMMI Radiopharm. Chem. 6, 33 (2021).
Sap, J. B. et al. Late-stage difluoromethylation: concepts, developments and perspective. Chem. Soc. Rev. 50, 8214–8247 (2021).
Buchner, E. & Curtius, T. Ueber die Einwirkung von Diazoessigäther auf aromatische Kohlenwasserstoffe. Ber. Dtsch. Chem. Ges. 18, 2377–2379 (1885).
Staudinger, H. & Kupfer, O. Über reaktionen des methylens. III. Diazomethan. Ber. Dtsch. Chem. Ges. 45, 501–509 (1912).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Geri, J. B. et al. Microenvironment mapping via Dexter energy transfer on immune cells. Science 367, 1091–1097 (2020).
Marinelli, M., Santini, C. & Pellei, M. Recent advances in medicinal applications of coinage-metal (Cu and Ag) N-heterocyclic carbene complexes. Curr. Top. Med. Chem. 16, 2995–3017 (2016).
Smith, C. A. et al. N-heterocyclic carbenes in materials chemistry. Chem. Rev. 119, 4986–5056 (2019).
Campbell, M. G. et al. Bridging the gaps in 18F PET tracer development. Nat. Chem. 9, 1–3 (2017).
Deng, X. et al. Chemistry for positron emission tomography: recent advances in 11C‐, 18F‐, 13N‐, and 15O‐labeling reactions. Angew. Chem. Int. Ed. 58, 2580–2605 (2019).
McCluskey, S. P., Plisson, C., Rabiner, E. A. & Howes, O. Advances in CNS PET: the state-of-the-art for new imaging targets for pathophysiology and drug development. Eur. J. Nucl. Med. Mol. Imaging 47, 451–489 (2020).
Prchalová, E., Štěpánek, O., Smrček, S. & Kotora, M. Medicinal applications of perfluoroalkylated chain-containing compounds. Future Med. Chem. 6, 1201–1229 (2014).
Huiban, M. et al. A broadly applicable [18F]trifluoromethylation of aryl and heteroaryl iodides for PET imaging. Nat. Chem. 5, 941–944 (2013).
Levin, M. D. et al. A catalytic fluoride-rebound mechanism for C(sp3)–CF3 bond formation. Science 356, 1272–1276 (2017).
van der Born, D. et al. A universal procedure for the [18F]trifluoromethylation of aryl iodides and aryl boronic acids with highly improved specific activity. Angew. Chem. Int. Ed. 53, 11046–11050 (2014).
Mizuta, S. et al. Catalytic decarboxylative fluorination for the synthesis of tri-and difluoromethyl arenes. Org. Lett. 15, 2648–2651 (2013).
Verhoog, S. et al. Silver-mediated 18F-labeling of aryl–CF3 and aryl–CHF2 with 18F-fluoride. Synlett 27, 25–28 (2016).
Shi, H. et al. Synthesis of 18F‐difluoromethylarenes from aryl (pseudo) halides. Angew. Chem. Int. Ed. 55, 10786–10790 (2016).
Yuan, G. et al. Metal-free 18F-labeling of aryl–CF2H via nucleophilic radiofluorination and oxidative C–H activation. Chem. Commun. 53, 126–129 (2017).
Sap, J. B. et al. Synthesis of 18F-difluoromethylarenes using aryl boronic acids, ethyl bromofluoroacetate and [18F]fluoride. Chem. Sci. 10, 3237–3241 (2019).
Zhao, Q. et al. Radiosynthesis of [18F]arylSCF2H using aryl boronic acids, S-(chlorofluoromethyl) benzenesulfonothioate and [18F]fluoride. CCS Chem. 3, 1921–1928 (2021).
Trump, L. et al. Late‐stage 18F‐difluoromethyl labeling of N‐heteroaromatics with high molar activity for PET imaging. Angew. Chem. Int. Ed. 58, 13149–13154 (2019).
Ni, C. & Hu, J. Recent advances in the synthetic application of difluorocarbene. Synthesis 46, 842–863 (2014).
Fier, P. S. & Hartwig, J. F. Synthesis of difluoromethyl ethers with difluoromethyltriflate. Angew. Chem. Int. Ed. 52, 2092–2095 (2013).
Xie, Q. et al. Efficient difluoromethylation of alcohols using TMSCF2Br as a unique and practical difluorocarbene reagent under mild conditions. Angew. Chem. Int. Ed. 56, 3206–3210 (2017).
Birchall, J. M., Haszeldine, R. N. & Roberts, D. W. Cyclopropane chemistry. Part II. Cyclopropanes as sources of difluorocarbene. J. Chem. Soc. Perkin Trans. I 1071–1078 (1973).
Jia, Y., Yuan, Y., Huang, J., Jiang, Z. & Yang, Z. Synthesis of difluorinated heterocyclics through metal-free [8+1] and [4+1] cycloaddition of difluorocarbene. Org. Lett. 23, 2670–2675 (2021).
Feng, Z., Min, Q. & Zhang, X. Access to difluoromethylated arenes by Pd-catalyzed reaction of arylboronic acids with bromodifluoroacetate. Org. Lett. 18, 44–47 (2016).
Deng, X., Lin, J. & Xiao, J. Pd-catalyzed transfer of difluorocarbene. Org. Lett. 18, 4384–4387 (2016).
Feng, Z., Min, Q., Fu, X., An, L. & Zhang, X. Chlorodifluoromethane-triggered formation of difluoromethylated arenes catalysed by palladium. Nat. Chem. 9, 918–923 (2017).
Fu, X. et al. Controllable catalytic difluorocarbene transfer enables access to diversified fluoroalkylated arenes. Nat. Chem. 11, 948–956 (2019).
Smail, T. & Rowland, F. S. Insertion reactions of mono-and difluorocarbene with hydrogen halides. J. Phys. Chem. 74, 1866–1871 (1970).
Prakash, G. S. et al. Long‐lived trifluoromethanide anion: a key intermediate in nucleophilic trifluoromethylations. Angew. Chem. Int. Ed. 53, 11575–11578 (2014).
Hine, J. & Porter, J. J. The formation of difluoromethylene from difluoromethyl phenyl sulfone and sodium methoxide. J. Am. Chem. Soc. 82, 6178–6181 (1960).
Xing, L. et al. Fluorine in drug design: a case study with fluoroanisoles. ChemMedChem 10, 715–726 (2015).
Khotavivattana, T. et al. 18F‐labeling of aryl–SCF3, –OCF3 and –OCHF2 with [18F]fluoride. Angew. Chem. Int. Ed. 54, 9991–9995 (2015).
Dahl, K., Halldin, C. & Schou, M. New methodologies for the preparation of carbon-11 labeled radiopharmaceuticals. Clin. Transl. Imaging 5, 275–289 (2017).
Pipal, R. W. et al. Metallaphotoredox aryl and alkyl radiomethylation for PET ligand discovery. Nature 589, 542–547 (2021).
Mullard, A. Deuterated drugs draw heavier backing. Nat. Rev. Drug Discov. 15, 219–222 (2016).
Cai, Z. et al. Synthesis and in vivo evaluation of [18F]UCB-J for PET imaging of synaptic vesicle glycoprotein 2A (SV2A). Eur. J. Nucl. Med. Mol. Imaging 46, 1952–1965 (2019).
Zheng, J., Winkeler, A., Peyronneau, M.-A., Dollé, F. & Boisgard, R. Evaluation of PET imaging performance of the TSPO radioligand [18F]DPA-714 in mouse and rat models of cancer and inflammation. Mol. Imaging Biol. 18, 127–134 (2016).
Keller, T. et al. Radiosynthesis and preclinical evaluation of [18F]F-DPA, a novel pyrazolo[1,5a]pyrimidine acetamide TSPO radioligand, in healthy Sprague Dawley rats. Mol. Imaging Biol. 19, 736–745 (2017).
Kuchar, M. & Mamat, C. Methods to increase the metabolic stability of 18F-radiotracers. Molecules 20, 16186–16220 (2015).
Lelos, M. J. & Dunnett, S. B. Generating Excitotoxic Lesion Models of Huntington’s Disease 209–220 (Springer, 2018).
Zhou, W. et al. Transition-metal difluorocarbene complexes. Chem. Commun. 57, 9316–9329 (2021).
Li, X. et al. Copper-mediated aerobic (phenylsulfonyl)difluoromethylation of arylboronic acids with difluoromethyl phenyl sulfone. Chem. Commun. 52, 3657–3660 (2016).
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Quality guidelines. ICH https://www.ich.org/page/quality-guidelines (2019).
Acknowledgements
This research has received funding from the Engineering and Physical Sciences Research Council (EP/V013041/1, J.B.I.S.), Pfizer, Janssen, UCB, the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement number 721902 (C.F.M., S.M.H. and T.A.M.). J.F. is grateful to the Centre for Doctoral Training in Synthesis for Biology and Medicine for a studentship, generously supported by GlaxoSmithKline, MSD, Syngenta and Vertex. J.B.I.S. acknowledges financial support from an EPSRC Doctoral Prize (EP/T517811/1). R.S.P. acknowledges the RMACC Summit supercomputer at the University of Colorado Boulder and Colorado State University, the Extreme Science and Engineering Discovery Environment (XSEDE) through allocation TG-CHE180056, and support from the National Science Foundation (NSF CHE-1955876). We thank B. G. Davis, S. Verhoog and T. C. Wilson for comments, and T. Khotavivattana for preliminary experiments.
Author information
Authors and Affiliations
Contributions
J.B.I.S. and C.F.M. labelled the tBu-substituted difluorocarbene reagent and performed all insertions and cycloadditions with this reagent. J.B.I.S., C.F.M. and J.F. prepared the substrates and performed all automation experiments. J.B.I.S. and J.F. performed the cross-coupling reactions, one-pot procedures, the synthesis of the radiotracer for the imaging study, the radiosynthesis of all difluorocarbene reagents, and the experiments with the Cl-substituted difluorocarbene reagent, and developed the NMR assay to probe isotopic dilution. J.B.I.S. and N.J.W.S. performed preliminary studies for the radiosynthesis of the tBu-substituted difluorocarbene reagent. A.B.D. and R.S.P. performed and analysed the computational studies. T.A.M. and C.F.M. did an initial metabolic stability study. J.B.I.S., J.F., M.J.L. and S.J.P. performed all the in vivo experiments. S.M.H. prepared selected substrates. J.B.I.S., J.F. and V.G. conducted the revisions. J.B.I.S., R.S.P. and V.G. wrote the manuscript. All authors read and commented on the paper. V.G. conceived and supervised the project.
Corresponding author
Ethics declarations
Competing interests
C.G. is an employee of UCB Pharma. C.W.a.E. is an employee of Pfizer Inc. A patent application (no. GB2113561.1; Difluorocarbene radiosynthesis) has been filed, from which V.G., J.B.I.S., C.F.M., M.T., N.J.W.S., S.M.H. and A.A.T. may benefit from royalties. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks John Groves and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains: Materials and methods, Supplementary Text, Figs. 1–69, Tables 1–14, NMR spectra and References.
Rights and permissions
About this article
Cite this article
Sap, J.B.I., Meyer, C.F., Ford, J. et al. [18F]Difluorocarbene for positron emission tomography. Nature 606, 102–108 (2022). https://doi.org/10.1038/s41586-022-04669-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04669-2
This article is cited by
-
Highlight selection of radiochemistry and radiopharmacy developments by editorial board
EJNMMI Radiopharmacy and Chemistry (2023)
-
Copper-catalysed difluorocarbene transfer enables modular synthesis
Nature Chemistry (2023)
-
Highlight selection of radiochemistry and radiopharmacy developments by editorial board
EJNMMI Radiopharmacy and Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.