Abstract
Colorectal cancer (CRC) is among the most frequent forms of cancer, and new strategies for its prevention and therapy are urgently needed1. Here we identify a metabolite signalling pathway that provides actionable insights towards this goal. We perform a dietary screen in autochthonous animal models of CRC and find that ketogenic diets exhibit a strong tumour-inhibitory effect. These properties of ketogenic diets are recapitulated by the ketone body β-hydroxybutyrate (BHB), which reduces the proliferation of colonic crypt cells and potently suppresses intestinal tumour growth. We find that BHB acts through the surface receptor Hcar2 and induces the transcriptional regulator Hopx, thereby altering gene expression and inhibiting cell proliferation. Cancer organoid assays and single-cell RNA sequencing of biopsies from patients with CRC provide evidence that elevated BHB levels and active HOPX are associated with reduced intestinal epithelial proliferation in humans. This study thus identifies a BHB-triggered pathway regulating intestinal tumorigenesis and indicates that oral or systemic interventions with a single metabolite may complement current prevention and treatment strategies for CRC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw sequencing data for this study are available at NCBI GEO under accession numbers PRJNA809364 (bulk RNA-seq), GSE196964 (single-cell RNA-seq), PRJNA808110 (ChIP–seq) and PRJNA809037 (bisulfite sequencing). Source data are provided with this paper.
References
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M. & Wallace, M. B. Colorectal cancer. Lancet 394, 1467–1480 (2019).
Brenner, H., Kloor, M. & Pox, C. P. Colorectal cancer. Lancet 383, 1490–1502 (2014).
Beyaz, S. et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53–58 (2016).
Ringel, A. E. et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell 183, 1848–1866 (2020).
Xu, J. et al. Association between markers of glucose metabolism and risk of colorectal cancer. BMJ Open 6, e011430 (2016).
Aggarwal, B. B. & Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 71, 1397–1421 (2006).
Di Tano, M. et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat. Commun. 11, 2332 (2020).
Weng, M. L. et al. Fasting inhibits aerobic glycolysis and proliferation in colorectal cancer via the Fdft1-mediated AKT/mTOR/HIF1α pathway suppression. Nat. Commun. 11, 1869 (2020).
Grivennikov, S. I. et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491, 254–258 (2012).
Ang, Q. Y. et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 181, 1263–1275 (2020).
Youm, Y. H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Newman, J. C. & Verdin, E. β-Hydroxybutyrate: a signaling metabolite. Annu. Rev. Nutr. 37, 51–76 (2017).
Hall, K. D. et al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat. Med. 27, 344–353 (2021).
Yamashita, K., Katoh, H. & Watanabe, M. The homeobox only protein homeobox (HOPX) and colorectal cancer. Int. J. Mol. Sci. 14, 23231–23243 (2013).
Takeda, N. et al. Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420–1424 (2011).
Wang, Y. et al. Long-term culture captures injury–repair cycles of colonic stem cells. Cell 179, 1144–1159 (2019).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Kaiko, G. E. et al. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165, 1708–1720 (2016).
Fenaux, P. et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 10, 223–232 (2009).
Thangaraju, M. et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Canc. Res. 69, 2826–2832 (2009).
Lien, E. C. et al. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 599, 302–307 (2021).
Chen, F. et al. Hop is an unusual homeobox gene that modulates cardiac development. Cell 110, 713–723 (2002).
Tunaru, S. et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9, 352–355 (2003).
Takeda, N. et al. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development 140, 1655–1664 (2013).
Becker, C., Fantini, M. C. & Neurath, M. F. High resolution colonoscopy in live mice. Nat. Protoc. 1, 2900–2904 (2006).
Miyoshi, H. & Stappenbeck, T. S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 8, 2471–2482 (2013).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Dessau, R. B. & Pipper, C. B. “R”–project for statistical computing. Ugeskr. Laeger 170, 328–330 (2008).
Huber, W. et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 12, 115–121 (2015).
Amorim, C. F. et al. Variable gene expression and parasite load predict treatment outcome in cutaneous leishmaniasis. Sci. Transl. Med. 11, eaax4204 (2019).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 4, 1521 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. EdgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Linderman, G. C. et al. Zero-preserving imputation of scRNA-seq data. Nat Commun. 13, 192 (2022).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Smillie, C. S. et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 178, 714–730 (2019).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Acknowledgements
We thank the members of the Levy and Thaiss laboratories for discussions and input. We acknowledge the University of Pennsylvania Molecular Pathology and Imaging Core, Department of Medicine, Gastroenterology Division, and the CDB Microscopy Core Facility for processing and imaging of tissue samples; S. Offermans, M. V. Kumar and N. Singh for providing mice; the Shalem laboratory (CHOP) for help with cell sorting; E. Krespan and D. Beiting (UPenn) for support with RNA-seq; P. W. Czyzewicz (Fox Chase Cancer Center) for assistance with animal experiments; X. Wang and A. Blanco (UPenn) for help with CRISPR experiments; J. Schug and the PSOM Next-Generation Sequencing Core for assistance with bisulfite sequencing analysis; E. Friedman and the Microbial Culture and Metabolomics Core of the PennCHOP Microbiome Program; and the Host Microbial Analytic and Repository Core of the Center for Molecular Studies in Digestive and Liver Diseases (P30DK050306). Human organoid support was from the Children’s Hospital of Philadelphia Gastrointestinal Epithelium Modelling Program. We thank J. H.-M. and S. Cherry for advice. J.A.E. is supported by NIH grant R35-HL-140018. R.J. is supported by NIH grant R01-HL-139783 and the Burroughs Wellcome Fund. A.C.W. is supported by training grant T32‐AI‐055400‐19. P.L. is supported by training grant T32-AI-141393. S.I.G. is supported by NIH grants R01-CA-218133, R01-CA-227629 and P30-CA-006927 and by the BSF US–Israel Foundation. J.K. is supported by a Boehringer Ingelheim Fonds MD fellowship. K.M.S. is supported by DFG Fellowship DFG SCHN 1626/1-1. C.A.T. is a Pew biomedical scholar and a Kathryn W. Davis Aging Brain scholar and is supported by the NIH Director’s New Innovator Award (DP2-AG-067492), the Agilent Early Career Professor Award, and grants from the Edward Mallinckrodt Jr Foundation, the IDSA Foundation, the Thyssen Foundation, PennCHOP Microbiome Program, Penn Institute for Immunology, Penn Center for Molecular Studies in Digestive and Liver Diseases (P30-DK-050306), Penn Skin Biology and Diseases Resource-based Center (P30-AR-069589), Penn Diabetes Research Center (P30-DK-019525) and Penn Institute on Aging. M.L. is supported by the NIH Director’s New Innovator Award (DP2-AG-067511), an American Cancer Society Scholar Award, the Searle Scholar program, the Edward Mallinckrodt Jr Foundation and grants from the Abramson Cancer Center, Penn Institute for Immunology, Penn Center for Molecular Studies in Digestive and Liver Diseases, Penn Center for Precision Medicine, Penn Institute on Aging, Penn Center of Excellence in Environmental Toxicology (P30-ES-013508) and the Borrelli Family Pilot Grant in Lynch Syndrome.
Author information
Authors and Affiliations
Contributions
O.D.-P. conceived the project, performed and analysed all in vivo and in vitro experiments, interpreted the results and wrote the manuscript. A.C.W. performed in vivo and in vitro experiments. A.M.G. and L.D. performed animal experiments. Z.C., Y.T., B.Y. and O.E. performed organoid experiments. G.E. performed ChIP–seq. J.L. performed bisulfite sequencing. J.K., B.M., S.K. and N.A. performed animal experiments. P.L. and N.L. analysed single-cell RNA-seq data. H.C.D. analysed RNA-seq data. Y.L. analysed ChIP–seq and bisulfite sequencing data. J.F. and K.M.S. performed statistical analysis. Q.W., J.A.E. and R.J. provided animals and insights. E.E.F. scored pathology slides. W.B. provided insights. J.R.K., K.E.H., K.H.K., S.B., C.J.L. and B.W.K. provided human organoids, cell lines and biopsies. S.L.B. supervised ChIP–seq experiments. M. Li supervised statistical analysis. S.I.G. provided insights and tools. C.A.T. and M. Levy conceived the project, mentored the participants, interpreted the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Charles Mackay and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The impact of ketogenic diets on intestinal tumour growth.
a–f, Representative colonoscopy images (a), colonoscopy-based tumour counts (b), representative histological images (c), histological tumour counts (d), representative macroscopic images (e), and tumour size quantification (f) of AOM/DSS-treated mice fed six different diets. g, Survival of AOM/DSS-treated mice fed a KD or control diet. h–j, Representative colonoscopy and histology images (h), colonoscopy-based tumour numbers (i) and histological tumour count (j) in CDX2CreERT-Apcfl/fl mice fed a KD or control diet. k–p, Representative colonoscopy images (k, n), colonoscopy-based tumour quantification (l, o), and tumour scores (m, p) from CDX2CreERT-Apcfl/fl mice fed a KD or control diet housed in different animal vivaria. q–t, Colonoscopy-based tumour scores of AOM/DSS-treated KD-fed mice in a treatment model initiated after tumorigenesis (q, r) and a cessation model with discontinued diet (s, t). Error bars indicate means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Exact n and p-values are presented in Supplementary Table 3.
Extended Data Fig. 2 The immunological and epithelial impact of ketogenic diets.
a, b, Continuous recording (a) and quantification (b) of energy intake in mice fed six different diets with the indicated fat content. Grey areas indicate the dark phase. c, Transcript levels of Il17a in colonic tumours of AOM/DSS-treated mice on KD and control diet. d–g, Representative colonoscopy and histology images (d), colonoscopy-based tumour quantification (e), tumour score by colonoscopy (f), and histological tumour counts (g) in Rag1−/− mice fed a KD or control diet. h–j, Representative colonoscopy images (h), colonoscopy-based tumour quantification (i) and tumour score (j) in CDX2CreERT-Apcfl/fl mice reconstituted with Nlrp3-deficient or -sufficient bone marrow. k–n, Representative images (k, m) and quantification (l, n) of Ki67-stained colons in healthy (k, l) and AOM/DSS-treated (m, n) KD-fed mice and controls. o, p, Representative immunohistochemistry images (o) and quantification (p) of Mcl1 staining of tumours from AOM/DSS-treated KD-fed mice and controls. Error bars indicate means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Exact n and p-values are presented in Supplementary Table 3.
Extended Data Fig. 3 Inhibition of intestinal epithelial growth by beta-hydroxybutyrate.
a–c, Schematic (a), representative images (b), and growth quantification (c) of intestinal organoids obtained from KD-fed mice and controls. d, e, Representative images (d) and growth quantification (e) of intestinal organoids after isolation of stem cells and Paneth cells from KD-fed mice and controls. f, Colonic concentrations of BHB in mice fed the indicated diets. g, h Viability assessments by flow cytometry (g) or luminescent ATP detection (h) of BHB-treated organoids and controls. i, j, Representative images (i) and size quantification (j) of organoids from CDX2CreERT-Apcfl/fl mice after culturing with the indicated concentrations of BHB. k, Ki67 quantification by flow cytometry in organoids treated with BHB. l, Size quantification of organoids grown in media containing different glucose concentrations, with or without BHB supplementation. Error bars indicate means ± SEM. Scale bars represent 68 µm. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Exact n and p-values are presented in Supplementary Table 3.
Extended Data Fig. 4 Inhibition of intestinal tumour growth by beta-hydroxybutyrate.
a, b, Representative macroscopic images (a) and representative histology images (b) of CDX2CreERT-Apcfl/fl mice fed either KD or control diet, with or without daily oral supplementation with BHB esters as indicated. c, Colonoscopy-based tumour quantification in AOM/DSS-treated mice receiving oral supplementation with either BHB or butyrate. d, BHB concentrations in the blood of AOM/DSS-treated mice on standard rodent diet receiving daily oral supplementation with BHB esters. e, f, Representative images (e) and quantification (f) of Ki67-stained tumours in AOM/DSS-treated mice supplemented with BHB. g–i, Representative colonoscopy images (g), colonoscopy-based tumour quantification (h), and tumour score by colonoscopy (i) in AOM/DSS-treated mice receiving daily oral supplementation with BHB esters. j, k, Macroscopic tumour counts (j) and representative macroscopic images (k) of Lgr5CreERT-Apcfl/fl mice receiving daily oral supplementation with BHB esters. l, Glucose concentrations in the blood of AOM/DSS-treated mice on standard chow receiving daily oral supplementation with BHB esters. m–q, Representative colonoscopy images (m), colonoscopy-based tumour quantification (n), representative histology images (o), histological tumour counts (p), and representative macroscopic images (q) of CDX2CreERT-Apcfl/fl mice supplemented with BHB salts by osmotic mini-pumps. Error bars indicate means ± SEM. Scale bars represent 135 µm. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Exact n and p-values are presented in Supplementary Table 3.
Extended Data Fig. 5 The role of Hopx in BHB-mediated tumour suppression.
a, PCA plot of global gene expression of BHB-treated and control organoids. b, Ki67 expression in organoids from wild-type and Hopx-deficient mice treated with BHB. c, Transcript levels of Hopx in organoids treated with 2-deoxyglucose (2-DG) or BHB. d, Size quantification of APKS organoids treated with 2-DG or BHB. e, Size quantification of organoids from wild-type or Hopx-deficient mice cultured at the indicated glucose concentrations, with or without BHB supplementation. f, Transcript levels of Hopx in the indicated tissues from KD-fed mice and controls. g, h, Representative images (g) and quantification (h) of GFP in the colons of Hopx-GFP reporter mice fed a KD or control diet. i–l, Representative colonoscopy images (i), macroscopic images (j), histological images (k), and colonoscopy-based tumour score (l) of AOM/DSS-treated Hopx-deficient and wild-type mice fed a KD or control diet. m–p, Blood glucose (m), circulating BHB (n), tumour number by colonoscopy (o), and colonoscopy-based tumour score (p) of Hopx-deficient mice and controls receiving BHB supplementation or 30% caloric restriction (CR). Error bars indicate means ± SEM. Scale bar represents 160 µm. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p<0.0001. Exact n and p-values are presented in Supplementary Table 3.
Extended Data Fig. 6 Regulation of epithelial proliferation by Hopx.
a, b, Heatmap (a) and KEGG pathway analysis (b) of differentially expressed genes determined by RNA-sequencing of AOM/DSS-treated Hopx-deficient mice and wild-type littermates on KD. c–f, Examples of genes upregulated in KD-fed AOM/DSS-treated Hopx-deficient mice compared to wild-type littermates. Error bars indicate means ± SEM. * p < 0.05, ** p < 0.01. Exact n and p-values are presented in Supplementary Table 3.
Extended Data Fig. 7 The impact of BHB on intestinal epithelial histone acetylation.
a, Model by which HOPX is induced through the HDAC-inhibitory activity of BHB. b, HDAC activity in organoids treated with BHB, with or without the pharmacological HDAC inhibitor vorinostat. c–e, Representative flow cytometry plot (c), relative proportion of H3K9ac (d), and normalized MFI of H3K9ac (e) in intestinal organoids treated with BHB. f, HDAC activity in intestinal epithelial cells from mice treated with BHB esters. g–i, Representative flow cytometry plot (g), relative proportion of H3K9ac (h), and normalized MFI of H3K9ac (i) in intestinal epithelial cells from KD-fed mice and controls. j, k, Representative immunofluorescence staining (j) and quantification (k) of pan-acetylation levels in colons from KD-fed mice and controls. l, Size of wild-type organoids treated with BHB and/or vorinostat. m, n, Size quantification of wild-type (m) and APKS organoids (n) exposed to BHB or butyrate. o, p, PCA plot (o) and k-means clustering (p) of global gene expression in organoids treated with BHB or the HDAC inhibitor (HDACi) vorinostat. q, Transcript abundance of Hopx determined by RNA-sequencing of organoids treated with BHB or vorinostat. r, Normalized profiles of H3K9ac in the Hopx locus from ChIP-sequencing of intestinal epithelial cells from mice treated with BHB or fed a 90% fat diet. s, Size of Hopx-deficient organoids after treatment with BHB and/or vorinostat. t, Size quantification of organoids from Hopx-deficient mice exposed to BHB or butyrate. u, Quantification of HDAC activity in wild-type and Hopx-deficient organoids treated with BHB and/or vorinostat. v, w, Tumour numbers (v) and representative colonoscopy images (w) in AOM/DSS-treated mice on KD and treated with the HDAC inhibitor vorinostat. Error bars indicate means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Exact n and p-values are presented in Supplementary Table 3.
Extended Data Fig. 8 The impact of BHB on intestinal epithelial DNA methylation.
a, Hypothetical model by which HOPX is induced through BHB-mediated methylation changes. b–d, PCA plots (b, c) and scatter plot (d) of bisulphite sequencing of intestinal epithelial cells from mice treated with BHB or fed a KD compared to controls. e, Heatmap of CpG methylation status in the Hopx locus determined by bisulphite sequencing of intestinal epithelial cells from mice treated with BHB or fed a 90% fat diet. f, Transcript abundance of Hopx in organoids treated with BHB and/or 5-azacitidine (5-Aza). g, h, Size of wild-type (g) and Hopx-deficient organoids (h) after treatment with BHB and/or 5-Aza. i, j, Images of wild-type (i) and Hopx-deficient (j) organoids treated with BHB and 5-Aza. Error bars indicate means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Exact n and p-values are presented in Supplementary Table 3.
Extended Data Fig. 9 A CRISPR-based approach to identify mediators of Hopx induction by BHB.
a, Hypothetical model by which HOPX is induced through BHB-mediated G protein-coupled receptor signalling. b–g, Expression of the indicated genes in organoids after CRISPR-mediated targeting thereof. h–m, Representative images (h) and size quantitation (i-m) of organoids treated with BHB after CRISPR-mediated targeting of the indicated genes. n, Schematic of organoid growth from Hcar2-deficient mice, cultured with BHB, butyrate, or vorinostat. o, Representative images of organoids from wild-type and Hcar2-deficient mice treated with BHB. p–v, Transcript levels of Hopx in organoids treated with BHB after CRISPR-mediated targeting of the indicated genes. Error bars indicate means ± SEM. **** p < 0.0001. Exact n and p-values are presented in Supplementary Table 3.
Extended Data Fig. 10 The impact of BHB on human organoids and colorectal cancer cell lines.
a, b, Representative images (a) and quantification (b) of organoids from a healthy paediatric donor after culturing with BHB. c, d, Representative images (c) and quantification (d) of organoids from a CRC patient after culturing with the indicated concentrations of BHB. e, EdU staining of HT-29 cells treated with the indicated concentrations of BHB. f, Cell cycle analysis of HT-29 cells treated with BHB. g, EdU staining of HT-29 cells cultured at the indicated concentrations of glucose, with or without addition of BHB. h, EdU staining of HCT116 cells treated with the indicated concentrations of BHB. i, j, Cell cycle analysis of Caco-2 cells (i) and RKO cells (j) treated with BHB. k, l, Expression of HCAR2 (g), and HOPX (h) by the indicated cell lines. Error bars indicate means ± SEM. * p < 0.05, *** p < 0.001, **** p < 0.0001. Exact n and p-values are presented in Supplementary Table 3.
Extended Data Fig. 11 The impact of BHB and HOPX on colorectal cancer biopsies.
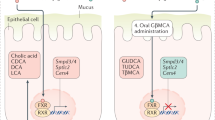
a, Workflow of analysis of CRC patient cohort. b, Serum BHB levels in 41 colon cancer patients. c, d, UMAP clustering (c) and marker gene expression (d) in single-cell RNA-sequencing data of colonic biopsies from nine CRC patients. e–h, Epithelial HOPX expression binned by serum BHB levels (e), correlation of average epithelial HOPX expression with serum BHB (f), correlation of epithelial cell cycle progression with serum BHB (g), and correlation of the proportion of cells in S phase with the proportion of HOPX-expressing epithelial cells (h) as determined by single-cell RNA-sequencing of colonic biopsies of tumour and normal tissue from nine CRC patients. Error bars indicate means ± SEM. The p- and R-values were determined by linear regression. i, Cartoon illustrating that ketogenic diet induces BHB, which in turn signals via HCAR2 to induce HOPX, decelerate proliferation of the colonic crypt, and suppress cancer development. This pathway is contrasted with the mechanisms engaged by other tumour-modulatory diets. Exact n and p-values are presented in Supplementary Table 3.
Supplementary information
Supplementary Information
This file contains Supplementary Fig. 1 and Supplementary Tables 1–3.
Source data
Rights and permissions
About this article
Cite this article
Dmitrieva-Posocco, O., Wong, A.C., Lundgren, P. et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature 605, 160–165 (2022). https://doi.org/10.1038/s41586-022-04649-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04649-6
This article is cited by
-
β-hydroxybutyrate inhibits malignant phenotypes of prostate cancer cells through β-hydroxybutyrylation of indoleacetamide-N-methyltransferase
Cancer Cell International (2024)
-
Dietary factors and their influence on immunotherapy strategies in oncology: a comprehensive review
Cell Death & Disease (2024)
-
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2024)
-
Systemic proteome adaptions to 7-day complete caloric restriction in humans
Nature Metabolism (2024)
-
Targeting apoptosis and unfolded protein response: the impact of β-hydroxybutyrate in clear cell renal cell carcinoma under glucose-deprived conditions
Molecular Biology Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.