Abstract
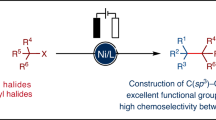
Recent research in medicinal chemistry has suggested that there is a correlation between an increase in the fraction of sp3 carbons—those bonded to four other atoms—in drug candidates and their improved success rate in clinical trials1. As such, the development of robust and selective methods for the construction of carbon(sp3)–carbon(sp3) bonds remains a critical problem in modern organic chemistry2. Owing to the broad availability of alkyl halides, their direct cross-coupling—commonly known as cross-electrophile coupling—provides a promising route towards this objective3,4,5. Such transformations circumvent the preparation of carbon nucleophiles used in traditional cross-coupling reactions, as well as stability and functional-group-tolerance issues that are usually associated with these reagents. However, achieving high selectivity in carbon(sp3)–carbon(sp3) cross-electrophile coupling remains a largely unmet challenge. Here we use electrochemistry to achieve the differential activation of alkyl halides by exploiting their disparate electronic and steric properties. Specifically, the selective cathodic reduction of a more substituted alkyl halide gives rise to a carbanion, which undergoes preferential coupling with a less substituted alkyl halide via bimolecular nucleophilic substitution to forge a new carbon–carbon bond. This protocol enables efficient cross-electrophile coupling of a variety of functionalized and unactivated alkyl electrophiles in the absence of a transition metal catalyst, and shows improved chemoselectivity compared with existing methods.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this work are available within the paper and its Supplementary Information.
References
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Choi, J. & Fu, G. C. Transition metal–catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Everson, D. A. & Weix, D. J. Cross-electrophile coupling: principles of reactivity and selectivity. J. Org. Chem. 79, 4793–4798 (2014).
Wang, X., Dai, Y. & Gong, H. Nickel-catalyzed reductive couplings. Top. Curr. Chem. 374, 43 (2016).
Lucas, E. L. & Jarvo, E. R. Stereospecific and stereoconvergent cross-couplings between alkyl electrophiles. Nat. Rev. Chem. 1, 0065 (2017).
Jana, R., Pathak, T. P. & Sigman, M. S. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 111, 1417–1492 (2011).
Weix, D. J. Methods and mechanisms for cross-electrophile coupling of Csp2 halides with alkyl electrophiles. Acc. Chem. Res. 48, 1767–1775 (2015).
Cherney, A. H. & Reisman, S. E. Nickel-catalyzed asymmetric reductive cross-coupling between vinyl and benzyl electrophiles. J. Am. Chem. Soc. 136, 14365–14368 (2014).
Zhang, P., Le, C. C. & MacMillan, D. W. C. Silyl radical activation of alkyl halides in metallaphotoredox catalysis: a unique pathway for cross-electrophile coupling. J. Am. Chem. Soc. 138, 8084–8087 (2016).
Ackerman, L. K. G., Lovell, M. W. & Weix, D. J. Multimetallic catalysed cross-coupling of aryl bromides with aryl triflates. Nature 524, 454–457 (2015).
Sanford, A. B. et al. Nickel-catalyzed alkyl−alkyl cross-electrophile coupling reaction of 1,3-dimesylates for the synthesis of alkylcyclopropanes. J. Am. Chem. Soc. 142, 5017–5023 (2020).
Qian, X., Auffrant, A., Felouat, A. & Gosmini, C. Cobalt-catalyzed reductive allylation of alkyl halides with allylic acetates or carbonates. Angew. Chem. Int. Ed. 50, 10402–10405 (2011).
Liu, J.-H. et al. Copper-catalyzed reductive cross-coupling of nonactivated alkyl tosylates and mesylates with alkyl and aryl bromides. Chem. Eur. J. 20, 15334–15338 (2014).
Yu, X., Yang, T., Wang, S., Xu, H. & Gong, H. Nickel-catalyzed reductive cross-coupling of unactivated alkyl halides. Org. Lett. 13, 2138–2141 (2011).
Xu, H., Zhao, C., Qian, Q., Deng, W. & Gong, H. Nickel-catalyzed cross-coupling of unactivated alkyl halides using bis(pinacolato)diboron as reductant. Chem. Sci. 4, 4022–4029 (2013).
Smith, R. T. et al. Metallaphotoredox-catalyzed cross-electrophile Csp3−Csp3 coupling of aliphatic bromides. J. Am. Chem. Soc. 140, 17433–17438 (2018).
Diccianni, J. B., Katigbak, J., Hu, C. & Diao, T. Mechanistic characterization of (xantphos)Ni(I)-mediated alkyl bromide activation: oxidative addition, electron transfer, or halogen-atom abstraction. J. Am. Chem. Soc. 141, 1788–1796 (2019).
Wang, J., Gong, Y., Sun, D. & Gong, H. Nickel-catalyzed reductive benzylation of tertiary alkyl halides with benzyl chlorides and chloroformates. Org. Chem. Front. 8, 2944–2948 (2021).
Chen, H., Jia, X., Yu, Y., Qian, Q. & Gong, H. Nickel-catalyzed reductive allylation of tertiary alkyl halides with allylic carbonates. Angew. Chem. Int. Ed. 56, 13103–13106 (2017).
Xue, W. et al. Nickel-catalyzed formation of quaternary carbon centers using tertiary alkyl electrophiles. Chem. Soc. Rev. 50, 4162–4184 (2021).
Clayden, J., Greeves, N. & Warren, S. Organic Chemistry 2nd edn (Oxford Univ. Press, 2012).
Vasudevan, D. Direct and indirect electrochemical reduction of organic halides in aprotic media. Russ. J. Electrochem. 41, 310–314 (2005).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Moeller, K. D. Synthetic applications of anodic electrochemistry. Tetrahedron 56, 9527–9554 (2000).
Cleary, J. A., Mubarak, M. S., Vieira, K. L., Anderson, M. R. & Peters, D. G. Electrochemical reduction of alkyl halides at vitreous carbon cathodes in dimethylformamide. J. Electroanal. Chem. Interfacial Electrochem. 198, 107–124 (1986).
Chaussard, J. et al. Use of sacrificial anodes in electrochemical functionalization of organic halides. Synthesis 1990, 369–381 (1990).
Nedelec, J. Y., Ait-Haddou-Mouloud, H., Folest, J. C. & Perichon, J. Electrochemical cross-coupling of alkyl halides in the presence of a sacrificial anode. J. Org. Chem. 53, 4720–4724 (1988).
Andrieux, C. P., Gallardo, I. & Saveant, J. M. Outer-sphere electron-transfer reduction of alkyl halides. A source of alkyl radicals or of carbanions? Reduction of alkyl radicals. J. Am. Chem. Soc. 111, 1620–1626 (1989).
Knochel, P. New approach to boron-stabilized organometallics. J. Am. Chem. Soc. 112, 7431–7433 (1990).
Matteson, D. S. α-Halo boronic esters: intermediates for stereodirected synthesis. Chem. Rev. 89, 1535–1551 (1989).
Schmidt, J., Choi, J., Liu, A. T., Slusarczyk, M. & Fu, G. C. A general, modular method for the catalytic asymmetric synthesis of alkylboronate esters. Science 354, 1265–1269 (2016).
Sun, S.-Z., Börjesson, M., MartinMontero, R. & Martin, R. Site-selective Ni-catalyzed reductive coupling of α-haloboranes with unactivated olefins. J. Am. Chem. Soc. 140, 12765–12769 (2018).
Sandford, C. & Aggarwal, V. K. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 53, 5481–5494 (2017).
Li, C. et al. Decarboxylative borylation. Science 356, eaam7355 (2017).
Fawcett, A. et al. Photoinduced decarboxylative borylation of carboxylic acids. Science 357, 283–286 (2017).
Yang, Y. et al. Practical and modular construction of C(sp3)‑rich alkyl boron compounds. J. Am. Chem. Soc. 143, 471–480 (2021).
Bera, S., Mao, R. & Hu, X. Enantioselective C(sp3)–C(sp3) cross-coupling of non-activated alkyl electrophiles via nickel hydride catalysis. Nat. Chem. 13, 270–277 (2021).
Hong, K., Liu, X. & Morken, J. P. Simple access to elusive α-boryl carbanions and their alkylation: an umpolung construction for organic synthesis. J. Am. Chem. Soc. 136, 10581–10584 (2014).
Kischkewitz, M., Okamoto, K., Mück-Lichtenfeld, C. & Studer, A. Radical-polar crossover reactions of vinylboron ate complexes. Science 355, 936–938 (2017).
Collins, B. S. L., Wilson, C. M., Myers, E. L. & Aggarwal, V. K. Asymmetric synthesis of secondary and tertiary boronic esters. Angew. Chem. Int. Ed. 56, 11700–11733 (2017).
Hofstra, J. L., Cherney, A. H., Ordner, C. M. & Reisman, S. E. Synthesis of enantioenriched allylic silanes via nickel-catalyzed reductive cross-coupling. J. Am. Chem. Soc. 140, 139–142 (2018).
Wang, L. et al. C–O functionalization of α-oxyboronates: a deoxygenative gem-diborylation and gem-silylborylation of aldehydes and ketones. J. Am. Chem. Soc. 139, 5257–5264 (2017).
Hazrati, H. & Oestreich, M. Copper-catalyzed double C(sp3)–Si coupling of geminal dibromides: ionic-to-radical switch in the reaction mechanism. Org. Lett. 20, 5367–5369 (2018).
Vasilopoulos, A., Krska, S. W. & Stahl, S. S. C(sp3)–H methylation enabled by peroxide photosensitization and Ni-mediated radical coupling. Science 372, 398–403 (2021).
McMillan, A. J. et al. Practical and selective sp3 C−H bond chlorination via aminium radicals. Angew. Chem. Int. Ed. 60, 7132–7139 (2021).
Bishop, J. L., Quinn, R. & Dyar, M. D. Spectral and thermal properties of perchlorate salts and implications for Mars. Am. Mineral. 99, 1580–1592 (2014).
Xue, W., Shishido, R. & Oestreich, M. Bench-stable stock solutions of silicon Grignard reagents: application to iron- and cobalt-catalyzed radical C(sp3)–Si cross-coupling reactions. Angew. Chem. Int. Ed. 57, 12141–12145 (2018).
Chan, C.-Y., Lepeshkov, I. N. & Khoo, K. H. (eds) Alkaline Earth Metal Perchlorates (Solubility Data Series, Pergamon, 1989).
Okoshi, M., Yamada, Y., Yamada, A. & Nakai, H. Theoretical analysis on de-solvation of lithium, sodium, and magnesium cations to organic electrolyte solvents. J. Electrochem. Soc. 160, A2160–A2165 (2013).
Shao, Y. et al. Coordination chemistry in magnesium battery electrolytes: how ligands affect their performance. Sci. Rep. 3, 3130 (2013).
Acknowledgements
Financial support was provided by NIGMS (R01GM134088; to S.L.), NSF Center for Synthetic Organic Electrochemistry (CHE-2002158; to K.A.S.), and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. S.L. is grateful to the Research Corporation for Science Advancement for a Cottrell Scholar Award. This study made use of the NMR facility supported by the NSF (CHE-1531632). XPS data were collected at the Molecular Materials Research Center in the Beckman Institute of the California Institute of Technology. We thank C. Yang for providing propargylic chloride substrates.
Author information
Authors and Affiliations
Contributions
S.L. and K.A.S. supervised the project. N.S. and D.L. provided guidance on the project. Wen Zhang and S.L. conceived the work. Wen Zhang, L.L., N.S., D.L. and S.L. designed the experiments. Wen Zhang. and L.L. conducted the synthetic experiments and mechanism studies. Wendy Zhang, S.D.W. and K.A.S. conducted the analysis of electrode passivation. Y.W., J.M. and J.R. conducted the density functional theory calculations. Wen Zhang, L.L., Wendy Zhang, K.A.S. and S.L. wrote the manuscript. N.S., D.L. and S.D.W. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Sections 1–20, including Supplementary text, data, tables and figures and NMR spectra data—see contents page for details.
Rights and permissions
About this article
Cite this article
Zhang, W., Lu, L., Zhang, W. et al. Electrochemically driven cross-electrophile coupling of alkyl halides. Nature 604, 292–297 (2022). https://doi.org/10.1038/s41586-022-04540-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04540-4
This article is cited by
-
Nickel-electrocatalysed C(sp3)–C(sp3) cross-coupling of unactivated alkyl halides
Nature Catalysis (2024)
-
Congested C(sp3)-rich architectures enabled by iron-catalysed conjunctive alkylation
Nature Catalysis (2024)
-
Interfacial tuning of electrocatalytic Ag surfaces for fragment-based electrophile coupling
Nature Catalysis (2024)
-
Electrophotocatalytic hydrogenation of imines and reductive functionalization of aryl halides
Nature Communications (2024)
-
Paired electrocatalysis unlocks cross-dehydrogenative coupling of C(sp3)-H bonds using a pentacoordinated cobalt-salen catalyst
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.