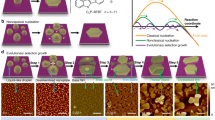
Abstract
The quality of crystalline two-dimensional (2D) polymers1,2,3,4,5,6 is intimately related to the elusive polymerization and crystallization processes. Understanding the mechanism of such processes at the (sub)molecular level is crucial to improve predictive synthesis and to tailor material properties for applications in catalysis7,8,9,10 and (opto)electronics11,12, among others13,14,15,16,17,18. We characterize a model boroxine 2D dynamic covalent polymer, by using in situ scanning tunnelling microscopy, to unveil both qualitative and quantitative details of the nucleation–elongation processes in real time and under ambient conditions. Sequential data analysis enables observation of the amorphous-to-crystalline transition, the time-dependent evolution of nuclei, the existence of ‘non-classical’ crystallization pathways and, importantly, the experimental determination of essential crystallization parameters with excellent accuracy, including critical nucleus size, nucleation rate and growth rate. The experimental data have been further rationalized by atomistic computer models, which, taken together, provide a detailed picture of the dynamic on-surface polymerization process. Furthermore, we show how 2D crystal growth can be affected by abnormal grain growth. This finding provides support for the use of abnormal grain growth (a typical phenomenon in metallic and ceramic systems) to convert a polycrystalline structure into a single crystal in organic and 2D material systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the paper and its Supplementary Information. Additional data are available from the corresponding authors upon reasonable request.
References
Wang, W. & Schlüter, A. D. Synthetic 2D polymers: a critical perspective and a look into the future. Macromol. Rapid Commun. 40, 1800719 (2019).
Feng, X. & Schlüter, A. D. Towards macroscopic crystalline 2D polymers. Angew. Chem. Int. Ed. Engl. 57, 13748–13763 (2018).
Servalli, M. & Schlüter, A. D. Synthetic two-dimensional polymers. Annu. Rev. Mater. Res. 47, 361–389 (2017).
Payamyar, P., King, B. T., Öttinger, H. C. & Schlüter, A. D. Two-dimensional polymers: concepts and perspectives. Chem. Commun. 52, 18–34 (2016).
Colson, J. W. & Dichtel, W. R. Rationally synthesized two-dimensional polymers. Nat. Chem. 5, 453–465 (2013).
Sakamoto, J., van Heijst, J., Lukin, O. & Schlüter, A. D. Two-dimensional polymers: just a dream of synthetic chemists? Angew. Chem. Int. Ed. Engl. 48, 1030–1069 (2009).
Wang, X. et al. Homochiral 2D porous covalent organic frameworks for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 138, 12332–12335 (2016).
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015).
Ding, S.-Y. et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 133, 19816–19822 (2011).
Dong, R. et al. Large-area, free-standing, two-dimensional supramolecular polymer single-layer sheets for highly efficient electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. Engl. 54, 12058–12063 (2015).
Dogru, M. et al. A photoconductive thienothiophene-based covalent organic framework showing charge transfer towards included fullerene. Angew. Chem. Int. Ed. Engl. 52, 2920–2924 (2013).
Wan, S., Guo, J., Kim, J., Ihee, H. & Jiang, D. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. Engl. 47, 8826–8830 (2008).
Li, X. et al. Tuneable near white-emissive two-dimensional covalent organic frameworks. Nat. Commun. 9, 2335 (2018).
DeBlase, C. R., Silberstein, K. E., Truong, T.-T., Abruña, H. D. & Dichtel, W. R. β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 135, 16821–16824 (2013).
Furukawa, H. & Yaghi, O. M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J. Am. Chem. Soc. 131, 8875–8883 (2009).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Liu, W. et al. A two-dimensional conjugated aromatic polymer via C–C coupling reaction. Nat. Chem. 9, 563–570 (2017).
Bin, H. et al. 11.4% Efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor. Nat. Commun. 7, 13651 (2016).
Liu, K. et al. On-water surface synthesis of crystalline, few-layer two-dimensional polymers assisted by surfactant monolayers. Nat. Chem. 11, 994–1000 (2019).
Lange, R. Z., Hofer, G., Weber, T. & Schlüter, A. D. A two-dimensional polymer synthesized through topochemical [2 + 2]-cycloaddition on the multigram scale. J. Am. Chem. Soc. 139, 2053–2059 (2017).
Kissel, P., Murray, D. J., Wulftange, W. J., Catalano, V. J. & King, B. T. A nanoporous two-dimensional polymer by single-crystal-to-single-crystal photopolymerization. Nat. Chem. 6, 774–778 (2014).
Kory, M. J. et al. Gram-scale synthesis of two-dimensional polymer crystals and their structure analysis by X-ray diffraction. Nat. Chem. 6, 779–784 (2014).
Schlüter, A. D. Mastering polymer chemistry in two dimensions. Commun. Chem. 3, 12 (2020).
Grill, L. & Hecht, S. Covalent on-surface polymerization. Nat. Chem. 12, 115–130 (2020).
Zhong, Y. et al. Wafer-scale synthesis of monolayer two-dimensional porphyrin polymers for hybrid superlattices. Science 366, 1379–1384 (2019).
Kissel, P. et al. A two-dimensional polymer prepared by organic synthesis. Nat. Chem. 4, 287–291 (2012).
Evans, A. M. et al. Seeded growth of single-crystal two-dimensional covalent organic frameworks. Science 361, 52–57 (2018).
Evans, A. M. et al. Emissive single-crystalline boroxine-linked colloidal covalent organic frameworks. J. Am. Chem. Soc. 141, 19728–19735 (2019).
Martínez-Abadía, M. & Mateo-Alonso, A. Structural approaches to control interlayer interactions in 2D covalent organic frameworks. Adv. Mater. 32, 2002366 (2020).
Li, H. et al. Nucleation–elongation dynamics of two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 142, 1367–1374 (2020).
Li, H. et al. Nucleation and growth of covalent organic frameworks from solution: the example of COF-5. J. Am. Chem. Soc. 139, 16310–16318 (2017).
Smith, B. J. & Dichtel, W. R. Mechanistic studies of two-dimensional covalent organic frameworks rapidly polymerized from initially homogenous conditions. J. Am. Chem. Soc. 136, 8783–8789 (2014).
Liu, X.-H. et al. On-surface synthesis of single-layered two-dimensional covalent organic frameworks via solid–vapor interface reactions. J. Am. Chem. Soc. 135, 10470–10474 (2013).
Liu, C., Yu, Y., Zhang, W., Zeng, Q. & Lei, S. Room-temperature synthesis of covalent organic frameworks with a boronic ester linkage at the liquid/solid interface. Chem. Eur. J. 22, 18412–18418 (2016).
Grossmann, L. et al. On-surface photopolymerization of two-dimensional polymers ordered on the mesoscale. Nat. Chem. 13, 730–736 (2021).
Schlüter, A. D., Weber, T. & Hofer, G. How to use X-ray diffraction to elucidate 2D polymerization propagation in single crystals. Chem. Soc. Rev. 49, 5140–5158 (2020).
Crawford, A. G. et al. Synthesis of 2- and 2,7-functionalized pyrene derivatives: an application of selective C–H borylation. Chem. Eur. J. 18, 5022–5035 (2012).
Wan, S., Guo, J., Kim, J., Ihee, H. & Jiang, D. A photoconductive covalent organic framework: self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chem. Int. Ed. Engl. 48, 5439–5442 (2009).
Medina, D. D. et al. Room temperature synthesis of covalent–organic framework films through vapor-assisted conversion. J. Am. Chem. Soc. 137, 1016–1019 (2015).
Dienstmaier, J. F. et al. Isoreticular two-dimensional covalent organic frameworks synthesized by on-surface condensation of diboronic acids. ACS Nano 6, 7234–7242 (2012).
Bilbao, N. et al. Anatomy of on-surface synthesized boroxine two-dimensional polymers. ACS Nano 14, 2354–2365 (2020).
Sassi, M., Oison, V., Debierre, J.-M. & Humbel, S. Modelling the two-dimensional polymerization of 1,4-benzene diboronic acid on a Ag surface. ChemPhysChem 10, 2480–2485 (2009).
Gasser, U., Weeks, E. R., Schofield, A., Pusey, P. N. & Weitz, D. A. Real-space imaging of nucleation and growth in colloidal crystallization. Science 292, 258–262 (2001).
Cai, Z.-F. et al. Electric-field-mediated reversible transformation between supramolecular networks and covalent organic frameworks. J. Am. Chem. Soc. 141, 11404–11408 (2019).
Zhan, G., Cai, Z.-F., Martínez-Abadía, M., Mateo-Alonso, A. & De Feyter, S. Real-time molecular-scale imaging of dynamic network switching between covalent organic frameworks. J. Am. Chem. Soc. 142, 5964–5968 (2020).
Li, D. et al. Direction-specific interactions control crystal growth by oriented attachment. Science 336, 1014–1018 (2012).
De Yoreo, J. J. et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349, aaa6760 (2015).
Viswanathan, R. & Bauer, C. L. Kinetics of grain boundary migration in copper bicrystals with [001] rotation axes. Acta Metall. 21, 1099–1109 (1973).
Sun, R. C. & Bauer, C. L. Tilt boundary migration in NaCl bicrystals. Acta Metall. 18, 639–647 (1970).
Rollett, A. D., Srolovitz, D. J. & Anderson, M. P. Simulation and theory of abnormal grain growth—anisotropic grain boundary energies and mobilities. Acta Metall. 37, 1227–1240 (1989).
Blum, V. et al. Ab initio molecular simulations with numeric atom-centered orbitals. Comput. Phys. Commun. 180, 2175–2196 (2009).
Marek, A. et al. The ELPA library: scalable parallel eigenvalue solutions for electronic structure theory and computational science. J. Phys. Condens. Matter 26, 213201 (2014).
Yu, V. W.-Z. et al. ELSI: a unified software interface for Kohn–Sham electronic structure solvers. Comput. Phys. Commun. 222, 267–285 (2018).
Tkatchenko, A., DiStasio, R. A., Car, R. & Scheffler, M. Accurate and efficient method for many-body van der Waals interactions. Phys. Rev. Lett. 108, 236402 (2012).
Ambrosetti, A., Reilly, A. M., DiStasio, R. A. Jr & Tkatchenko, A. Long-range correlation energy calculated from coupled atomic response functions. J. Chem. Phys. 140, 18A508 (2014).
Bitzek, E., Koskinen, P., Gähler, F., Moseler, M. & Gumbsch, P. Structural relaxation made simple. Phys. Rev. Lett. 97, 170201 (2006).
Hjorth Larsen, A. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Jorgensen, W. L. & Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 110, 1657–1666 (1988).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Hourahine, B. et al. DFTB plus, a software package for efficient approximate density functional theory based atomistic simulations. J. Chem. Phys. 152, 124101 (2020).
Grimme, S., Bannwarth, C. & Shushkov, P. A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86). J. Chem. Theory Comput. 13, 1989–2009 (2017).
Rüger, R. et al. AMS v.2021.1 (SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, the Netherlands, 2021); http://www.scm.com
Bannwarth, C. et al. Extended tight-binding quantum chemistry methods. WIREs Comput. Mol. Sci. 11, e1493 (2021).
Spicher, S. & Grimme, S. Robust atomistic modeling of materials, organometallic, and biochemical systems. Angew. Chem. Int. Ed. Engl. 59, 15665–15673 (2020).
Acknowledgements
We thank L. Verstraete and N. Bilbao for helpful discussions. We thank Y. Liao for helping with the preparation of the manuscript. We thank Y. Zhang for helping with data analysis. This work was supported by the Research Foundation - Flanders (FWO), in part by FWO under EOS 30489208, the Marie Sklodowska Curie ETN project ULTIMATE (GA-813036), and KU Leuven Internal Grant 3E180504, China Scholarship Council (201908350094), the Basque Foundation for Science (Ikerbasque), POLYMAT, the University of the Basque Country (Grupo de Investigación GIU17/054), Diputación Foral de Guipuzkoa, Gobierno Vasco (PIBA and BERC programmes), Gobierno de España (Ministerio de Economía y Competitividad CTQ2016-77970-R). Technical and human support provided by SGIker of UPV/EHU and European funding (ERDF and ESF) is acknowledged. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 722951). This project has received funding from the European Union´s Horizon 2020 research and innovation programme under grant agreement no. 664878 and no. 899895. In addition, support through the project IF/00894/2015, the advanced computing CPCA/A2/2524/2020 granting access to the Navigator cluster at LCA-UC and within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 & UIDP/50011/2020 funded by national funds through the Portuguese Foundation for Science and Technology I.P./MCTES is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
G.Z., Z.-F.C. and S.D.F. conceived the idea and designed the experiments. G.Z., Z.-F.C., L.Y. and N.H. carried out the in situ STM experiments and analysed the data. K.S. and M.M.-F carried out the DFT and tight-binding calculation. M.M.-A. carried out the synthesis of the monomer. The text was initially composed by G.Z., Z.-F.C., A.M.-A. and S.D.F., and all authors further contributed to the discussion of the experimental work and the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Markus Lackinger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Text 1–4 and Figs. 1–30.
Supplementary Data 1
Statistical analysis of critical nucleation size for Fig. 2e.
41586_2022_4409_MOESM3_ESM.mp4
Supplementary Video 1 In situ STM showing the dynamic nucleation process at liquid–solid interface with a scan rate of 1.5 min per frame. The video shows the emergence and elongation of some 2DPs nuclei within the disordered area. The video also demonstrates the disappearance of on-surface generated 2DPs nuclei, implying the reversible boroxine chemistry on the HOPG surface. The video is played at ×180 speed. Image size: 80 nm × 80 nm.
41586_2022_4409_MOESM4_ESM.mp4
Supplementary Video 2 In situ STM showing the nucleation–elongation processes. The video is played at ×180 speed. Image size: 150 nm × 150 nm.
41586_2022_4409_MOESM5_ESM.mp4
Supplementary Video 3 In situ STM showing the restricted motion of 2DPs nuclei, with the presence of surface defect. The video is played at ×180 speed. Image size: 60 nm × 60 nm.
41586_2022_4409_MOESM6_ESM.mp4
Supplementary Video 4 In situ STM showing the slow kinetics during normal grain growth. The video is played at ×180 speed. Image size: 60 nm × 60 nm.
41586_2022_4409_MOESM7_ESM.mp4
Supplementary Video 5 In situ STM showing the preferential growth of some nuclei (S2). During abnormal grain growth (AGG), S2 grows at the expense of R1 and S1. The video is played at ×180 speed. Image size: 100 nm × 100 nm.
41586_2022_4409_MOESM8_ESM.mp4
Supplementary Video 6 In situ STM showing the formation of a unidirectional domain (S2) by taking advantage of abnormal grain growth. The video is played at ×180 speed. Image size: 80 nm × 80 nm.
Rights and permissions
About this article
Cite this article
Zhan, G., Cai, ZF., Strutyński, K. et al. Observing polymerization in 2D dynamic covalent polymers. Nature 603, 835–840 (2022). https://doi.org/10.1038/s41586-022-04409-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04409-6
This article is cited by
-
Non-classical crystallization in soft and organic materials
Nature Reviews Materials (2024)
-
A self-standing three-dimensional covalent organic framework film
Nature Communications (2023)
-
Growth of single-crystal imine-linked covalent organic frameworks using amphiphilic amino-acid derivatives in water
Nature Chemistry (2023)
-
Covalent organic frameworks
Nature Reviews Methods Primers (2023)
-
In-situ imaging of strain-induced enhancement of hydrogen evolution activity on the extruded MoO2 sheets
Nano Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.