Abstract
Integration of sensory and molecular inputs from the environment shapes animal behaviour. A major site of exposure to environmental molecules is the gastrointestinal tract, in which dietary components are chemically transformed by the microbiota1 and gut-derived metabolites are disseminated to all organs, including the brain2. In mice, the gut microbiota impacts behaviour3, modulates neurotransmitter production in the gut and brain4,5, and influences brain development and myelination patterns6,7. The mechanisms that mediate the gut–brain interactions remain poorly defined, although they broadly involve humoral or neuronal connections. We previously reported that the levels of the microbial metabolite 4-ethylphenyl sulfate (4EPS) were increased in a mouse model of atypical neurodevelopment8. Here we identified biosynthetic genes from the gut microbiome that mediate the conversion of dietary tyrosine to 4-ethylphenol (4EP), and bioengineered gut bacteria to selectively produce 4EPS in mice. 4EPS entered the brain and was associated with changes in region-specific activity and functional connectivity. Gene expression signatures revealed altered oligodendrocyte function in the brain, and 4EPS impaired oligodendrocyte maturation in mice and decreased oligodendrocyte–neuron interactions in ex vivo brain cultures. Mice colonized with 4EP-producing bacteria exhibited reduced myelination of neuronal axons. Altered myelination dynamics in the brain have been associated with behavioural outcomes7,9,10,11,12,13,14. Accordingly, we observed that mice exposed to 4EPS displayed anxiety-like behaviours, and pharmacological treatments that promote oligodendrocyte differentiation prevented the behavioural effects of 4EPS. These findings reveal that a gut-derived molecule influences complex behaviours in mice through effects on oligodendrocyte function and myelin patterning in the brain.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data analysed for this study are included in this published Article and its Supplementary Information. Additional 2DG data are available online (https://gin.g-node.org/bneedham/Needham_Nature_2022). The WoL database annotation files are publicly available at GitHub (https://biocore.github.io/wol/download). The Greengenes database is publicly available online (https://greengenes.secondgenome.com/). Source data are provided with this paper.
Code availability
Custom scripts used in the fUSi analysis are available at GitHub (https://github.com/brittanyneedham/Needham_Nature2022).
References
Sharon, G. et al. Specialized metabolites from the microbiome in health and disease. Cell Metab. 20, 719–730 (2014).
Swann, J. R. et al. Application of 1H NMR spectroscopy to the metabolic phenotyping of rodent brain extracts: a metabonomic study of gut microbial influence on host brain metabolism. J. Pharm. Biomed. Anal. 143, 141–146 (2017).
Vuong, H. E., Yano, J. M., Fung, T. C. & Hsiao, E. Y. The microbiome and host behavior. Annu. Rev. Neurosci. 40, 21–49 (2017).
Needham, B. D., Kaddurah-Daouk, R. & Mazmanian, S. K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat. Rev. Neurosci. 21, 717–731 (2020).
Huang, F. & Wu, X. Brain neurotransmitter modulation by gut microbiota in anxiety and depression. Front. Cell Dev. Biol. 9, 649103 (2021).
Gacias, M. et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. eLife 5, e13442 (2016).
Hoban, A. E. et al. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 6, e774 (2016).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
Pan, S., Mayoral, S. R., Choi, H. S., Chan, J. R. & Kheirbek, M. A. Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci. 23, 487–499 (2020).
Maheras, K. J. et al. Absence of claudin 11 in CNS myelin perturbs behavior and neurotransmitter levels in mice. Sci. Rep. 8, 3798 (2018).
Bonnefil, V. et al. Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice. eLife 8, e40855 (2019).
Galvez-Contreras, A. Y., Zarate-Lopez, D., Torres-Chavez, A. L. & Gonzalez-Perez, O. Role of oligodendrocytes and myelin in the pathophysiology of autism spectrum disorder. Brain Sci. 10, 951 (2020).
Cassoli, J. S. et al. Disturbed macro-connectivity in schizophrenia linked to oligodendrocyte dysfunction: from structural findings to molecules. npj Schizophrenia 1, 15034 (2015).
Needham, B. D. et al. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol. Psychiatry 89, 451–462 (2020).
Gamage, N. et al. Human sulfotransferases and their role in chemical metabolism. Toxicol. Sci. 90, 5–22 (2006).
Chamkha, M., Garcia, J. L. & Labat, M. Metabolism of cinnamic acids by some Clostridiales and emendation of the descriptions of Clostridium aerotolerans, Clostridium celerecrescens and Clostridium xylanolyticum. Int. J. Syst. Evol. Microbiol. 51, 2105–2111 (2001).
Santamaría, L., Reverón, I., Felipe, F. L. D., Rivas, B. D. L. & Muñoz, R. Ethylphenol Formation by Lactobacillus plantarum: identification of the enzyme involved in the reduction of vinylphenols. Appl. Environ. Microbiol. 84, e01064-18 (2018).
Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Cresols (CDC, 2008); https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=946&tid=196
Penzo, M. A. et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015).
Ahrens, S. et al. A central extended amygdala circuit that modulates anxiety. J. Neurosci. 38, 5567–5583 (2018).
Duval, E. R., Javanbakht, A. & Liberzon, I. Neural circuits in anxiety and stress disorders: a focused review. Ther. Clin. Risk Manag. 11, 115–126 (2015).
Robinson, O. J., Pike, A. C., Cornwell, B. & Grillon, C. The translational neural circuitry of anxiety. J. Neurol. Neurosurg. Psychiatry 90, 1353–1360 (2019).
Barson, J. R., Mack, N. R. & Gao, W.-J. The paraventricular nucleus of the thalamus is an important node in the emotional processing network. Front. Behav. Neurosci. 14, 598469 (2020).
Kirouac, G. J. The paraventricular nucleus of the thalamus as an integrating and relay node in the brain anxiety network. Front. Behav. Neurosci. 15, 627633 (2021).
Park, H.-C. & Appel, B. Delta-notch signaling regulates oligodendrocyte specification. Development 130, 3747–3755 (2003).
Wang, S. et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63–75 (1998).
Williamson, J. M. & Lyons, D. A. Myelin Dynamics Throughout Life: An Ever-Changing Landscape? Front. Cell. Neurosci. 12, 424 (2018).
Holschneider, D. P., Wang, Z. & Pang, R. D. Functional connectivity-based parcellation and connectome of cortical midline structures in the mouse: a perfusion autoradiography study. Front. Neuroinform. 8, 61 (2014).
Kaller, M. S., Lazari, A., Blanco-Duque, C., Sampaio-Baptista, C. & Johansen-Berg, H. Myelin plasticity and behaviour—connecting the dots. Curr. Opin. Neurobiol. 47, 86–92 (2017).
Barak, B. et al. Neuronal deletion of Gtf2i, associated with Williams syndrome, causes behavioral and myelin alterations rescuable by a remyelinating drug. Nat. Neurosci. 22, 700–708 (2019).
Xie, D. et al. Clemastine improves hypomyelination in rats with hypoxic–ischemic brain injury by reducing microglia-derived IL-1β via P38 signaling pathway. J. Neuroinflammation 17, 57 (2020).
Kirouac, G. J. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci. Biobehav. Rev. 56, 315–329 (2015).
Sharon, G., Sampson, T. R., Geschwind, D. H. & Mazmanian, S. K. The central nervous system and the gut microbiome. Cell 167, 915–932 (2016).
Tian, P., Wang, G., Zhao, J., Zhang, H. & Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 66, 43–51 (2019).
Koyama, H. et al. Effects of housing conditions on behaviors and biochemical parameters in juvenile cynomolgus monkeys (Macaca fascicularis). Exp. Anim. 68, 195–211 (2019).
Tian, J.-S. et al. A GC-MS urinary quantitative metabolomics analysis in depressed patients treated with TCM formula of Xiaoyaosan. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1026, 227–235 (2016).
Sankowski, B. et al. Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with Parkinson’s disease. Clin. Chim. Acta 501, 165–173 (2020).
Gabriele, S. et al. Urinary p-cresol is elevated in young French children with autism spectrum disorder: a replication study. Biomarkers 19, 463–470 (2014).
Neul, J. L. et al. Metabolic signatures differentiate Rett syndrome from unaffected siblings. Front. Integr. Neurosci. 14, 7 (2020).
Takesada, M., Miyamoto, E., Kakimoto, Y., Sano, I. & Kaneko, Z. Phenolic and indole amines in the urine of schizophrenics. Nature 207, 1199–1200 (1965).
Sun, C.-Y. et al. p-Cresol sulfate caused behavior disorders and neurodegeneration in mice with unilateral nephrectomy involving oxidative stress and neuroinflammation. Int. J. Mol. Sci. 21, 6687 (2020).
Franklin, K. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Elsevier Science, 2007).
Holdeman, L. V. & Moore, W. E. C. Anaerobe Laboratory Manual 4th edn (Virginia Polytechnic Institute and State University, 1977).
Martens, E. C., Chiang, H. C. & Gordon, J. I. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 4, 447–457 (2008).
Koropatkin, N. M., Martens, E. C., Gordon, J. I. & Smith, T. J. Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure 16, 1105–1115 (2008).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Banoglu, E. & King, R. S. Sulfation of indoxyl by human and rat aryl (phenol) sulfotransferases to form indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 27, 135–140 (2002).
Teubner, W., Meinl, W., Florian, S., Kretzschmar, M. & Glatt, H. Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem. J. 404, 207–215 (2007).
Sharon, G. et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177, 1600–1618 (2019).
Iadecola, C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42 (2017).
Mace, E. et al. Functional ultrasound imaging of the brain: theory and basic principles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 60, 492–506 (2013).
Tiran, E. et al. Transcranial functional ultrasound imaging in freely moving awake mice and anesthetized young rats without contrast agent. Ultrasound Med. Biol. 43, 1679–1689 (2017).
Needham, B. D. et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. GitHub https://github.com/brittanyneedham/Needham_Nature2022 (2022).
Demené, C. et al. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases doppler and fultrasound sensitivity. IEEE Trans. Med. Imaging 34, 2271–2285 (2015).
Osmanski, B.-F., Pezet, S., Ricobaraza, A., Lenkei, Z. & Tanter, M. Functional ultrasound imaging of intrinsic connectivity in the living rat brain with high spatiotemporal resolution. Nat. Commun. 5, 5023 (2014).
Armstrong, R. A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 34, 502–508 (2014).
Holschneider, D. P., Guo, Y., Wang, Z., Vidal, M. & Scremin, O. U. Positive allosteric modulation of cholinergic receptors improves spatial learning after cortical contusion injury in mice. J. Neurotrauma 36, 2233–2245 (2019).
Sokoloff, L. Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J. Cereb. Blood Flow Metab. 1, 7–36 (1981).
Wang, Z., Pang, R. D., Hernandez, M., Ocampo, M. A. & Holschneider, D. P. Anxiolytic-like effect of pregabalin on unconditioned fear in the rat: an autoradiographic brain perfusion mapping and functional connectivity study. Neuroimage 59, 4168–4188 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Xu, X., Wells, A. B., O’Brien, D. R., Nehorai, A. & Dougherty, J. D. Cell type-specific expression analysis to identify putative cellular mechanisms for neurogenetic disorders. J. Neurosci. 34, 1420–1431 (2014).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Zambon, A. C. et al. GO-Elite: a flexible solution for pathway and ontology over-representation. Bioinformatics 28, 2209–2210 (2012).
Tyszka, J. M., Readhead, C., Bearer, E. L., Pautler, R. G. & Jacobs, R. E. Statistical diffusion tensor histology reveals regional dysmyelination effects in the shiverer mouse mutant. Neuroimage 29, 1058–1065 (2006).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Yeh, F.-C. et al. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE 8, e80713 (2013).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Mastronarde, D. N. Correction for non-perpendicularity of beam and tilt axis in tomographic reconstructions with the IMOD package. J. Microsc. 230, 212–217 (2008).
Carranza-Torres, I. E. et al. Organotypic culture of breast tumor explants as a multicellular system for the screening of natural compounds with antineoplastic potential. BioMed Res. Int. 2015, 618021 (2015).
Leger, M. et al. Object recognition test in mice. Nat. Protoc. 8, 2531–2537 (2013).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480 (2016).
Komada, M., Takao, K. & Miyakawa, T. Elevated plus maze for mice. J. Vis. Exp. https://doi.org/10.3791/1088 (2008).
Takao, K. & Miyakawa, T. Light/dark transition test for mice. J. Vis. Exp. https://doi.org/10.3791/104 (2006).
Miedel, C. J., Patton, J. M., Miedel, A. N., Miedel, E. S. & Levenson, J. M. Assessment of spontaneous alternation, novel object recognition and limb clasping in transgenic mouse models of amyloid-β and Tau neuropathology. J. Vis. Exp 28, 55523 (2017).
Shih, H.-T. & Mok, H.-K. ETHOM: event-recording computer software for the study of animal behavior. Acta Zool. Taiwanica 11, 47–61 (2000).
Acknowledgements
We thank the members of the Mazmanian laboratory for evaluating the manuscript; S. Reisman and L. Chapman (Caltech) for synthesizing 4EPS; the staff at the Caltech Kavli Nanoscience Institute for help with maintaining the TF30 electron microscope, and the Gordon and Betty Moore and Beckman Foundations for gifts to Caltech to support electron microscopy. Funding: this work was supported by funds from the Center for Environmental Microbial Interactions to B.D.N., National Science Foundation MRI grant 1920364 for Bruker console upgrading, the Human Frontier Science Program (grant no. LT000217/2020-C) to C.R., the National Institutes of Health (NIH) (2 P50 GM082545-08) to P.J.B., the Ministry of Science and Technology in Taiwan (MOST107-2320-B-006-072-MY3; MOST108-2321-B-006-025-MY2) to W.-L.W., and the Heritage Medical Research Institute, L. and B. Fetter, and the NIH (MH100556 and AG063744) to S.K.M.
Author information
Authors and Affiliations
Contributions
Conceptualization: B.D.N. and S.K.M. Methodology: B.D.N., M.F., M.D.A., Z.W., W.-L.W., J.H., M.S.L., J.A.G., C.R., S.-J.H. and D.P.H. Formal analysis: B.D.N., M.D.A., Z.W. and J.H. Investigation: B.D.N., M.F., M.D.A., Z.W., W.-L.W., J.C.B., C.R., J.H., S.-J.H., Q.Z., M.S.L. and Y.G. Biochemical pathway investigation and strain engineering: M.F. and M.A.F. Gene abundance analysis: Q.Z., J.C.B. and R.K. fUSi imaging: C.R., J.C.B., B.D.N. and M.G.S. 2DG analysis: Z.W., B.D.N., Y.G. and D.P.H. QuantSeq analysis: J.H., W.-L.W., B.D.N. and D.H.G. Oligodendrocyte characterization: B.D.N., M.D.A., J.C.B., M.S.L. and J.A.G. ET: M.S.L., B.D.N. and M.D.A. MRI/diffusion tensor imaging: S.-J.H. and B.D.N. Animal behaviour: B.D.N. and M.D.A. Resources: P.J.B., D.G., D.P.H., M.A.F., R.K., M.G.S. and S.K.M. Writing original draft: B.D.N. Writing review and editing: B.D.N., M.F., M.D.A., Z.W., W.-L.W., J.H., M.S.L., J.A.G., D.P.H., M.A.F. and S.K.M. Visualization: B.D.N., M.F., M.D.A., Z.W., W.-L.W., J.H., J.C.B., C.R., S.-J.H., M.S.L. and M.A.F. Supervision: S.K.M. Project administration: B.D.N. Funding acquisition: B.D.N., W.-L.W., P.J.B. and S.K.M.
Corresponding authors
Ethics declarations
Competing interests
S.K.M. has financial interests in Axial Biotherapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Gabriel Corfas, Jane Foster, Robert Quinn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Strain engineering and 4EPS quantification.
a, Strain legend corresponding to Fig. 1b–d, containing further details for strain names and cloning strategy. Additional information can be found in the Methods and Supplementary Tables 1–3. b–e, Maps for integration vectors. b, The BO1194 gene was cloned into the pNBU2 vector with an erythromycin resistant marker under the phage promoter. c, The PAD gene was cloned into pNBU2 vector with an erythromycin resistant marker under the phage promoter. d, The PAD gene was cloned into the pNBU2 vector with a tetracycline resistant marker under the phage promoter. e, BO1194 and PAD were tandemly connected, putting the phage promoter in front of both genes, then icloned into the pNBU2 vector with the erythromycin resistant marker. f, Creatinine corrected urinary levels of 4EPS from SPF, GF, gnotobiotic mice colonized with strains as labelled, and a mouse model of atypical behaviour including anxiety-like phenotypes. This data corresponds to abbreviated data in Fig. 1f. SPF, specific pathogen free mice; BO, Bacteroides ovatus mono-colonized mice; 4EP−, GF mice colonized with non-producing strain pair (B. ovatus Δ1194 and WT L. plantarum with endogenous VPR) used throughout; WT, GF mice colonized with wild type strain pair (WT B. ovatus and WT L. plantarum with endogenous VPR) before optimal engineering; 4EP+, colonized with strain pair engineered for higher production of 4EP+ (B. ovatus+1194/PAD and WT L. plantarum with endogenous VPR), used throughout; CNTNAP2, conventionally colonized mouse model of atypical behaviour (left to right columns: n=15, 5, 5, 9, 7, 9, 11). g, 4EPS levels (creatinine corrected) in urine of wild type, specific pathogen free (SPF) mice fed one of two isocaloric diets matched for protein, mineral, carbohydrate, and fat levels but differing in either high tyrosine fish meal (fish diet), or a high plant protein (soy diet) (n=8 each group)p=0.002. h, 4EPS levels (creatinine corrected) in urine of gnotobiotic mice colonized with isogenic strain pairs of B. ovatus and wild type L. plantarum, which differ only in the presence or absence of gene 1194, and thus the tyrosine lyase activity requires to convert tyrosine to p-coumaric acid (n=2 each group). i, Alignments of genes used to engineer the 4EP synthesis pathway to the reference genomes in the WoL database, showing that these genes are found in ~25 genomes each, most of which are common human gut lineages. j, 4EP and 4EPS levels (ug/ml) in serum of colonized mice (n=7 each group). k, 4EP+ and 4EP− colonized mice were injected with the organic anion transporter to inhibit potential 4EPS transport out of the brain, then analysed by LCMS (n=6 each group). Extended Data Fig. 1g. l, Conventionally colonized (SPF) mice were injected with probenecid and then injected with high dose 4EPS (n=8) or saline vehicle (n=8), and whole brain lysate was analysed by LCMS compared to 4EPS injection alone (n=4)p<0.0001. m, Conventionally colonized (SPF) mice were injected with probenecid and then gavaged with 4EP, then whole brains were harvested at 30-minute intervals and 4EPS levels were quantified by LCMS (n=4; vehicle group n=3)p=0.01. Two independent trials on biological replicates were used for the experiments in the figure. Abbreviations: SPF, specific pathogen-free; GF, germ-free; BO, Bacteroides ovatus; WT, wild type; LP, Lactobacillus plantarum. Data represent mean ± SEM analysed by a two-tailed Welch’s t-test or one-way ANOVA with Dunnett multiple comparisons test as appropriate. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤0.0001.
Extended Data Fig. 2 4EP sulfation, experimental timeline, normal weight gain, colonization and intestinal barrier of 4EP+ mice.
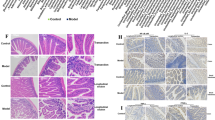
a, In vitro recombinant SULT1A1 sulfotransferase assay with 4EP as potential sulfate acceptor (n=5)p<0.0001. b, Results of the sulfotransferase assay using various recombinant SULTs (n=3)p<0.0001. c, 4EP sulfation capacity of cytosolic fractions of brain, colon, liver, and small intestinal tissue, each containing endogenous sulfotransferases (n=2 datapoints of samples pooled from triplicate biological replicates, with only 1 pool for SI). Ion intensity of 4EPS measured by LCMS is plotted along the y-axis. d, Confirmation of expression of the Sult1a1 gene measured by qPCR in tissue from the brain, colon, liver, and small intestine of colonized mice (n=8; brain n=6)p<0.0001. e, Schematic of mouse experimental timeline, showing ages of mice at colonization of GF mice with 4EP+/− strains, behaviour testing and tissue collection. f, Weights of mice (grams) after colonization of GF mice with 4EP− or 4EP+ bacteria (n=13). g, Ambulatory activity of 4EP− and 4EP+ mice over ten minutes, measured by distance moved when mice were placed in an open arena and allowed to explore (4EP− n=21; 4EP+ n=24). h, FITC-dextran levels in serum as a measure of intestinal permeability (4EP− n=5, 4EP+ n=6). i, Faecal output of colonized mice over 10 min (n=13). j, Images of hematoxylin and eosin (H&E) stained small intestine and colon of 4EP+/− mice (representative images of n=4). Scale bar 50μm. k, Colonization of ex-GF mice with engineered Bacteroides ovatus (BO) and Lactobacillus plantarum (LP), plotted as colony forming units (CFU) per gram of intestinal contents (n=4). l, Quantification of bacterial distance from the intestinal epithelium imaged by electron tomography, where each data point represents a separate animal comprised of an average of 5-10 bacterial cells per image per mouse (4EP− n=4; 4EP+ n=5). m, Example images of bacterial cells near the intestinal epithelial layer of 4EP+/− mice. Two independent trials using multiple randomized litters were used for the experiments in the figure. Abbreviations: SULT, sulfotransferase; SI, small intestine. Data represent mean ± SEM. Panel f was analysed by a 2-way ANOVA with a Bonferroni multiple comparison correction. Panels g-i and l were analysed using two-tailed Welch’s t-tests and panels a-d, k by a one-way ANOVA with Dunnett multiple comparisons test.
Extended Data Fig. 3 Lack of inflammatory signals in peripheral and brain cytokine and immune profiles of 4EP+/− mice.
a, b, Cytokine and chemokine levels presented in bar graph where grey (−) is 4EP− and green (+) is 4EP+, measured by bioplex in colon (n=9) a, and serum (n=9) b. No significant differences were observed. c–d, Flow cytometry of spleens of 4EP+ and 4EP− mice. For gating strategy see Supplementary Fig. 2. c, Percentages of CD4+ or CD8+ T cells (4EP− n=11, 4EP+ n=10). d, Percentage of B cells (4EP− n=11, 4EP+ n=10)p<0.0001. e, Cytokine and chemokine levels presented in bar graph where grey (−) is 4EP− and green (+) is 4EP+, measured by bioplex (n=9) in brain tissue (Il-3 p=0.046; Il-5 p=0.01; KC p=0.02; TNF-a p=0.01). f, Flow cytometry percentages of microglia in the brain of 4EP− and 4EP+ mice. For gating strategy see Supplementary Fig. 2. g, Relative expression of microglial genes in microglial-enriched samples (n=8). Multiple randomized litters were used for the experiments in the figure. Each data point represents biologically independent mice from multiple randomized litters examined over one (a-b) or two (c-h) respective experiments. Data represent mean ± SEM. Panels a, b, d, f, and h were analysed by one-way ANOVA with Dunnett multiple comparisons test comparing each 4EP+/4EP− pair, and panels g and e by a two-tailed Welch’s t-test. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001.
Extended Data Fig. 4 Extended information on functional connectivity experiments fUSi and autoradiography.
a, Schematic representation of the functional ultrasound (fUSi) set-up. Functional acquisitions are acquired non-invasively through intact skull and scalp in anesthetized mice during 15 min per coronal plane. Created with BioRender.com. b, Maps overlaid with regions of interest coloured in a gradient for easier visualization. Significant pairs are indicated, corresponding to the data in main Fig. 2b. Three coronal planes per mouse were studied: Bregma -0.9 mm, Bregma -1.6 mm, Bregma -2mm. 50, 52 and 52 ROIS are respectively delineated for each plane according the Paxinos Atlas. Coronal plane B-0.9mm: ROIs #1 to #8 are located in the left cortex, ROI#9 is the left hippocampus, ROIs#11 to #21 are located in the thalamus, ROI#22 is the right hippocampus, ROIs#23 to #30 are located in the right cortex and finally ROIs#31 to #48 are subthalamic regions. Coronal plane B-1.6mm: ROIs #1 to #20 are located in the cortex, ROIs#21 #22 are the left and right hippocampi, ROIs#23 to #38 are located in the thalamus and ROIs#39 to #42 are subthalamic regions. Coronal plane B-2mm: ROIs #1 to #9 are located in the left cortex, ROI#10 is the left hippocampus, ROIs#11 to #22 are located in the thalamus, ROI#23 is the right hippocampus, ROIs#24 to #32 are located in the right cortex and finally ROIs#33 to #50 are subthalamic regions. c–d, Colour-coded overlays over representative coronal (c) and sagittal (d) sections of the mouse brain template showing significant differences in regional cerebral glucose uptake following open field exposure in 4EP+ mice compared to 4EP− mice (n=11) (t-test, p ≤ 0.05, extent threshold > 200 contiguous voxels, with both conditions met to be deemed significant; red/blue: increase/decrease in glucose uptake in 4EP+ compared to 4EP− mice). e, Quantitated 2DG-uptake by region of interest, including the amygdala, hypothalamus and PVT, confirming changes in relative 2DG uptake in the open field groups (n=11 each group) From left to right, p=0.01; 0.03; 0.02; 0.009; 0.003). Average optical density of each ROI in each animal was normalized to whole-brain average of that animal. Abbreviations: AM, amygdala; HY, hypothalamus; PVT, paraventricular nucleus of the thalamus; BNST, bed nucleus of the stria terminalis; S1, primary somatosensory cortex; CPu, caudate putamen; Hb, habenular nucleus; RT, reticular nucleus of the thalamus; VPL/VPM, Posterior Lateral/Ventral Posterior Medial Thalamus; R, right; L, left. Two cohorts of mice from multiple litters were used for each experiment in this figure. Panel e was analysed by one-way ANOVA with multiple comparison correction and two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli. * p ≤ 0.05.
Extended Data Fig. 5 Extended results of mRNA high throughput sequencing (QuantSeq), and oligodendrocyte analysis by immunofluorescence implicating oligodendrocyte differences in 4EP+ mice.
a, Principal component analysis of all samples analysed by QuantSeq, with tight clustering by brain region. Each brain region is coloured according to the legend. b, Differential gene analysis summary showing number of significantly different genes in 6 tested brain regions. Top pie chart refers to total number of differential genes by one-way group ANOVA with all the 4EP− and 4EP+, at baseline and post-EPM. The bottom pie chart was generated according to the specific significant (p<0.05) contrasts between 4EP+ and 4EP− conditions of post-EPM conditions as calculated with the post-hoc Tukey HSD test. c–d, GO terms enriched in genes that are upregulated (c) or downregulated (d) in the PVT in 4EP+ relative to 4EP− mice in the baseline, home cage condition. X-axis represents -log10(P) of enrichment analysis. e, p-value histogram of PVT sequencing at baseline, graphed as p-values binned by 0.05 along the x-axis and number of genes along the y-axis. f, Adjusted p-values for 4EP+/4EP− comparison of expression of oligodendrocyte specific genes in all 6 brain regions analysed by QuantSeq, corresponding to data presented in Main Fig. 3c for the PVT. p<0.05 in bold. g, PVT seed analysis correlating 2DG uptake in the PVT to the rest of the brain. Images show 3D rendered, average whole brain correlation data (n=11). The centre of the PVT is indicated at the cross section of the red lines, and the number of negatively correlated voxels is shown below in each. Significance is illustrated according to the legend. A minimum threshold of 200 contiguous voxels with p<0.05 was used. h, Schematic of immature OPC and mature, myelinating oligodendrocyte markers used for immunostaining, flow cytometry, or western blots in this study. i, Raw counts of mature oligodendrocytes, measured by CC1+ and OLIG2+ staining in 4EP+ and 4EP− mice in the PVT, used to calculate maturity quotient in Fig. 3f (n=8). p=0.04. j, Raw counts of immature oligodendrocytes, measured by NG2+ and OLIG2+ staining in 4EP+ and 4EP− mice in the PVT, used to calculate maturity quotient in Fig. 3f (n=8 each group). p=0.055. k, Representative images (corresponding to main Fig. 3e, f) of the PVT in brain sections from 4EP− and 4EP+ mice, stained for OLIG2, using total cumulative counts from 3-5 images per replicate (n=8). Scale bar 100 µm. l, Raw counts of OLIG2+ cells in the PVT of 4EP+/− mice. Each data point represents an individual mouse, with total cumulative counts from the PVT (n=8) p=0.8. m, Raw counts of NeuN+ staining in the PVT (4EP− n=10, 4EP+ n=9), using average counts from 3-5 images per replicate. n, Representative images of pan-neuronal NeuN staining in the PVT, with 3-5 images per replicate (4EP− n=10, 4EP+ n=9). Scale bar 100 µm. o, NG2+ staining in extended regions of the brain (n=3 each group) p=0.009. Abbreviations: PVT, the paraventricular nucleus of the thalamus; BNST, bed nucleus of the stria terminalis; BLA, basolateral amygdala; mPFC, medial prefrontal cortex; HY, hypothalamus; vHPC, ventral hippocampus; OLIG2, oligodendrocyte transcription factor 2; NG2, neural/glial antigen 2; CC1, antibody (anti-adenomatous polyposis coli (APC) clone) that binds mature oligodendrocyte marker; MOG, myelin oligodendrocyte glycoprotein; MBP, myelin basic protein; ACA, anterior commissure; CC, corpus callosum; LHB, lateral habenula; LS, lateral septum; MHB, medial habenula; MS, medial septum; ME, median eminence; PFC, prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus; SM, stria medullaris of thalamus. Two cohorts of mice from multiple litters were used for experiments in panels g-o in this figure. Data represent mean ± SEM. Analysis performed using two-tailed Welch’s t-tests (i-m) or one-way ANOVA with Dunnett multiple comparison between 4EP+/− pairs (o). * p ≤ 0.05.
Extended Data Fig. 6 Extended results of oligodendrocyte analysis implicating oligodendrocyte differences in 4EP+ mice in vivo and in vitro.
a, Extended quantitation of flow cytometry with MOG+/NG2+ ratio by quadrant in the cerebellum, cortex, and hypothalamus (n=4). b–c, Western blot analysis of MOG (p=0.03) and MBP (p=0.002) markers (respectively) of mature oligodendrocytes from the PVT brain region. PVT punches from two mice were pooled per data point in quantitative data (n=6 pooled samples each group). For gel source data, see Supplementary Fig. 1. d–g, Organotypic brain slices were cultured in the presence of 10 uM 4EPS. d, Representative images of CC1, NG2 and Olig2 staining. Two cohorts of mice were used, with each data point in Fig 3i representing quantified images from samples from individual mice. Scale bar 20 µm. e, Example high-magnification image of axon in organotypic brain slices, stained with antibodies specific to NF (red), and PLP (green), with DAPI (blue), taken from image set used for quantification in 3i and Extended Data Fig. 6d. Scale bar 8 µm. f, qPCR results of oligodendrocyte genes, Cspg4 (p=0.04), Mog (p=0.03), and Mpb from organotypic brain slices (n=7). g, Western blot image of mature oligodendrocyte marker MBP (quantified in main Fig. 3l). For gel source data, see Supplementary Fig. 1. Abbreviations: NG2, neural/glial antigen 2; CC1, antibody (anti-adenomatous polyposis coli (APC) clone) that binds mature oligodendrocyte marker; MOG, myelin oligodendrocyte glycoprotein; MBP, myelin basic protein. Two cohorts of mice from multiple litters were used for experiments in this figure. Data represent mean ± SEM. Statistics were performed using two-tailed Welch’s t-tests. * p ≤ 0.05; ** p ≤ 0.01.
Extended Data Fig. 7 Extended results of myelin analysis by electron microscopy and DTI.
a, Additional ET of myelinated axons in 4EP− (top) and 4EP+ (bottom) mice visualized longitudinally along an axon (n=3; 4 images each). These longitudinal images were used only for qualitative visual assessment of myelin to accompany the other quantitative measures. b, Axon diameters (measured from cell membrane, not including myelin layer) from all axons used to calculate g-ratio (4EP−, 56; 4EP+, 70 axons) (n=4; 4 images each). p=0.1. c, g-ratio (r/R), the inner axon diameter/outer diameter of the myelin sheath, of 4EP+ and 4EP− mice. Each data point represents a mouse (n=4), which is an average of the g-ratio of all axons quantified from 4 images per mouse, where a larger g-ratio indicates a thinner layer of myelin. p=0.046. d, Plot of g-ratio (r/R) on the y-axis and axon diameter on the x-axis, with linear regression noted by lines (4EP−, 56; 4EP+, 70 axons) (n=4; 4 images each). e, Average g-ratio of each animal, binned by axon size, indicating that mid-sized range of axons are driving the change in overall g-ratio phenotype observed in the mice (n=4). f–g, Defined regions of interest for the PVT and corpus callosum (CC) overlaid on representative sagittal MRI image. h–i, representative tracts observed from a bilateral coronal plane view (left) and fractional anisotropy (FA) analysis (right) for the corpus callosum (CC), and whole brain (p=0.009), respectively (n=4). Two independent trials using multiple litters were used for experiments in this figure. Data represent mean ± SEM. Statistics were performed using two-tailed Welch’s t-tests (b,c,h,i), simple linear regression test (d), or one-way ANOVA with Dunnett multiple comparison between 4EP+/− groups at each size group. * p ≤ 0.05; ** p ≤ 0.01.
Extended Data Fig. 8 Additional behaviour tests in 4EP− and 4EP+ mice.
a, Distance traveled (p=0.06), time in centre (p=0.01), and thigmotaxis time (p=0.007), during open field test over a period of 10 min (distance traveled data was also shown in Extended Data Fig. 2g) (4EP− n=21, 4EP+ n=24). b, Time spent in the open arms of the elevated plus maze (EPM) (left)(p=0.02) and ratio of time spent in the open/time spent in the closed arms of the EPM (right) (4EP− n=21, 4EP+ n=24). c, Light/dark box: time mice spent in the open, lit portion of the arena. Test time, 10 min (4EP− n=25, 4EP+ n=23) p=0.02. d, Grooming: total time mice spent self-grooming over a period of 10 min (4EP− n=27, 4EP+ n=24). e, Social interaction: with an unfamiliar, age-matched male intruder. Total time socializing (left) and percent of total socializing that is anogenital sniffing (right) (4EP− n=25, 4EP+ n=22) p=0.0009. f, Ultrasonic vocalization: time spent vocalizing to an unfamiliar, age-matched female for 3 min (4EP− n=23, 4EP+ n=21) p=0.01. g, Novel object recognition (NOR): time spent investigating a novel object when presented with a novel and a familiar object (n=22). h, Y-maze alternations: percent of times mice repeated entry into an arm it had just visited rather than alternate to all arms in succession (4EP− n=15, 4EP+ n=16). i, Beam traversal: time required for the mouse to cross the narrowing beam (4EP− n=19, 4EP+ n=22). j, Pole descent: time required for the mouse to descend from the pole to the home cage (4EP− n=19, 4EP+ n=22). k, Wire hang: time the mouse hung on and explored the underside of the wire grid before releasing into cage below (4EP− n=19, 4EP+ n=22). l, Faecal 16s profiles of 4EP+/− mice after behaviour tests, where tan is the colonization group and contamination from exposure to behaviour tests is coloured according to the legend (left panel) and a magnified view of contaminants only (right panel) (4EP− n=8, 4EP+ n=10). Two independent cohorts of mice from multiple litters were used for each experiment in this figure. Data a-k represent mean ± SEM analysed by two-tailed Welch’s t-test. * p ≤ 0.05; ** p ≤ 0.01.
Extended Data Fig. 9 Behaviour results from 4EP or 4EPS administration by drinking water and anxiety behaviour tests of 4EP+/− mice relative to GF and SPF mice.
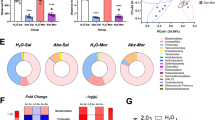
a–f, Behaviour tests of conventionally colonized mice administered 4EP or 4EPS by drinking water. a, Left panel: EPM, time spent in open arms over time spent in closed arms (seconds). Right panel: time spent at terminus (outer 1/3 of open arms)(Veh. n=15; 4EPS n=17; 4EP n=16) (Open/closed: Veh. vs 4EPS p<0.0001; Veh vs 4EP p=0.007; 4EPS vs 4EP p=0.03; Terminus: Veh vs 4EPS p=0.004). b, Open field test, from left to right: time spent in the centre area over thigmotaxis, time spent in centre (seconds), distance moved in open field test, showing an increase in activity in 4EP-treated animals (Veh. n=16; 4EPS n=16; 4EP n=18) p=02. c, Grooming test, total percent of time spent grooming, showing an increase in grooming in the 4EPS-treated animals (Veh. n=16; 4EPS n=18; 4EP n=17). d, Number of marbles buried (Veh. n=16; 4EPS n=17; 4EP n=18). e, Social interaction. Left panel: total socialization time (seconds). Right panel: % of total socialization time spent as anogenital sniffing (Veh. n=8; 4EPS n=7; 4EP n=7). f, USV test. Left panel: Vocalization events. Right panel: Total duration spent vocalizing (seconds) (Veh. n=16; 4EPS n=15; 4EP n=18). g, 4EPS levels in urine of mice administered 4EP or 4EPS in the drinking water (creatinine corrected)(Veh. n=12; 4EPS n=9; 4EP n=11)(Veh. vs 4EPS p=0.0005; Veh vs 4EP p=0.008; 4EPS vs 4EP p=0.03). h, 4EPS levels in serum of mice administered 4EP or 4EPS in the drinking water (Veh. n=4; 4EPS n=4; 4EP n=3). i, Quantified flow cytometry data of PVT of mice administered 4EP or 4EPS in the drinking water, with ratio of MOG+ quartile percentages/NG2+ quartile percentages presented (Veh. n=5; 4EPS n=5; 4EP n=4) p=0.03. For gating strategy see Supplementary Fig. 2 and main Fig. 3h. j, Quantified western blot data for MOG and MBP in the PVT of mice administered 4EP or 4EPS in the drinking water, with blots shown below (n=4 each group). (MBP: H2O vs 4EPS p=0.04; vs 4EP p=0.02). For gel source data, see Supplementary Figure 1. k–m, Anxiety behaviour tests contextualizing 4EP+/− mice to GF and SPF mice. k, Open field test, from left to right: time spent in the centre area over time spent along walls (4EP− vs 4EP+ p=0.04; 4EP+ vs GF p=0.02; 4EP+ vs SPF p=0.04), time spent in the centre (seconds), distance moved in open field test(4EP+ vs SPF p=0.047; GF vs SPF p-0.01) (4EP− n=8; 4EP+ n=9; GF n=9; SPF n=11). l, Elevated plus maze, time spent in the open arms/time spent in the closed arms ration (left)(4EP− vs 4EP+ p=0.03; 4EP+ vs SPF p=0.03) and time spent at the terminus (right) (4EP− n=9; 4EP+ n=9; GF n=11; SPF n=13). m, Marble burying (4EP− n=9; 4EP+ n=9; GF n=9; SPF n=112)(4EP− vs 4EP+ p=0.02; 4EP− vs SPF p=0.03; 4EP+ vs GF p=0.02; GF vs SPF p=0.03). Abbreviations: Veh., vehicle control; MOG, myelin oligodendrocyte glycoprotein; MBP, myelin basic protein. Two independent cohorts of mice from multiple litters were used for the experiments in this figure. Data represent mean ± SEM analysed by one-way ANOVA with Dunnett multiple comparison between all groups. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤0.0001.
Extended Data Fig. 10 Fluorescence imaging and extended behaviour test results of clemastine fumarate and miconazole-treated mice, and preliminary behaviour tests performed on both sexes, used to determine continued studies on males for the study.
a–i, 4EP+/− mice were administered vehicle (control), clemastine fumarate (clem.), or miconazole and then behaviour tested and imaged. a, Representative images of CC1 (green), NG2 (red), and OLIG2 (blue) staining in 4EP+/− mice with or without treatment. Representative of group of individual mice used for quantification: 4EP− control=7, treated=10; 4EP+ control=6, treated=6, which came from two cohorts (Scale bar 100 µm). b, Quantification of fluorescent imaging, presented as a ratio of CC1+/NG2+ cells (from left to right n=7, 10, 6, 6, 4, 4). The first four columns are also shown in Main Fig. 4i (Cont 4EP− vs 4EP+ p=0.04; 4EP+Cont vs 4EP+Clem p=0.01). c–d, Confirmation that treatment does not reduce 4EPS levels with clemastine (c) (Control. 4EP− n=4; Control. 4EP+ n=6, Clem 4EP− n=3; Clem 4EP+ n=4) or miconazole (d) (Control. 4EP− n=4; Control. 4EP+ n=4, Mic 4EP− n=4; Mic 4EP+ n=5) treatment. e, Extended EPM results (Control 4EP− n=13; Control 4EP+ n=13; Clem 4EP− n=12; Clem 4EP+ n=14). f, Extended open field results (Control 4EP− n=15; Control 4EP+ n=17; Clem 4EP− n=17; Clem 4EP+ n=17). g–i, Behavioural results for mice treated with miconazole, including g, Open field (Control 4EP− n=24; Control 4EP+ n=25; Mic. 4EP− n=18; Mic. 4EP+ n=20)(left graph: 4EP− Cont vs 4EP+ Cont p=0.02; 4EP+cont vs 4EP+Mic p=0.008)(middle graph: 4EP− Cont vs 4EP+ Cont p=0.02; 4EP+cont vs 4EP+Mic p=0.005). h, EPM (Control 4EP− n=24; Control 4EP+ n=25; Mic. 4EP− n=15; Mic. 4EP+ n=17)(left graph: 4EP− Cont vs 4EP+ Cont p=0.002; 4EP+cont vs 4EP+Mic p=0.03)(right graph: 4EP− Cont vs 4EP+Cont p=0.0009). i, Marble Burying (Control 4EP− n=24; Control 4EP+ n=26; Mic. 4EP− n=21; Mic. 4EP+ n=21)(p=0.001) j, Left panel: EPM, time spent in open arms over time spent in closed arms. Right panel: time spent at terminus (outer 1/3 of open arms) (Males 4EP− n=17; Males 4EP+ n=17; Females 4EP− n=20; Females 4EP+ n=21)p=0.02. k, Open field test, time spent in the centre area over time spent in thigmotaxis left), time spent in the centre (right) (Males 4EP− n=16; Males 4EP+ n=14; Females 4EP− n=18; Females 4EP+ n=13)p-values left to right: 0.005, 0.003. l, Number of marbles buried in marble burying test (Males 4EP− n=24; Males 4EP+ n=23; Females 4EP− n=17; Females 4EP+ n=26)p=0.03. Abbreviations: NG2, neural/glial antigen 2; CC1, antibody (anti-adenomatous polyposis coli (APC) clone) that binds mature oligodendrocyte marker; OLIG2, oligodendrocyte transcription factor 2; clem, clemastine fumarate; mic, miconazole. Two independent cohorts of mice from multiple litters were used for each experiment in this figure. Data represent mean ± SEM analysed by two-way ANOVA with Dunnett multiple comparison to 4EP+ group (panels b-i) or Sidak multiple comparison between 4EP+/− groups within each sex (panels j-l). * p ≤ 0.05, ** p ≤ 0.01.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, Supplementary Tables 1–6 and the captions for the Supplementary Tables.
Source data
Rights and permissions
About this article
Cite this article
Needham, B.D., Funabashi, M., Adame, M.D. et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 602, 647–653 (2022). https://doi.org/10.1038/s41586-022-04396-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04396-8
This article is cited by
-
The gut metabolite indole-3-propionic acid activates ERK1 to restore social function and hippocampal inhibitory synaptic transmission in a 16p11.2 microdeletion mouse model
Microbiome (2024)
-
Effects of radiofrequency field from 5G communication on fecal microbiome and metabolome profiles in mice
Scientific Reports (2024)
-
Host–microbiome orchestration of the sulfated metabolome
Nature Chemical Biology (2024)
-
Autism spectrum disorders and the gastrointestinal tract: insights into mechanisms and clinical relevance
Nature Reviews Gastroenterology & Hepatology (2024)
-
Amelioration of the brain structural connectivity is accompanied with changes of gut microbiota in a tuberous sclerosis complex mouse model
Translational Psychiatry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.