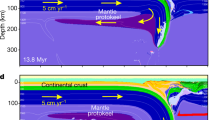
Abstract
The Hadean eon, following the global-scale melting of the mantle1,2,3, is expected to be a dynamic period, during which Earth experienced vastly different conditions. Geologic records, however, suggest that the surface environment of Earth was already similar to the present by the middle of the Hadean4,5. Under what conditions a harsh surface environment could turn into a habitable one remains uncertain6. Here we show that a hydrated mantle with small-scale chemical heterogeneity, created as a result of magma ocean solidification, is the key to ocean formation, the onset of plate tectonics and the rapid removal of greenhouse gases, which are all essential to create a habitable environment on terrestrial planets. When the mantle is wet and dominated by high-magnesium pyroxenites, the removal of carbon dioxide from the atmosphere is expected to be more than ten times faster than the case of a pyrolitic homogeneous mantle and could be completed within 160 million years. Such a chemically heterogeneous mantle would also produce oceanic crust rich in olivine, which is reactive with ocean water and promotes serpentinization. Therefore, conditions similar to the Lost City hydrothermal field7,8,9 may have existed globally in the Hadean seafloor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data are provided in the paper. Codes to reproduce the results are available at https://github.com/yoshi-miyazaki/Hadean-evolution/. The Gibbs energy minimization code is available at https://github.com/yoshi-miyazaki/GibbsE-minimization/. Source data are provided with this paper.
References
Matsui, T. & Abe, Y. Evolution of an impact-induced atmosphere and magma ocean on the accreting Earth. Nature 319, 303–305 (1986).
Tonks, W. B. & Melosh, H. J. Magma ocean formation due to giant impacts. J. Geophys. Res. 98, 5319–5333 (1993).
Canup, R. M. & Asphaug, E. Origin of the Moon in a giant impact near the end of the Earth’s formation. Nature 412, 708–712 (2001).
Wilde, S. A., Valley, J. W., Peck, W. H. & Graham, C. M. Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago. Nature 409, 175–178 (2001).
Harrison, T. M. The Hadean crust: Evidence from >4 Ga zircons. Annu. Rev. Earth Planet. Sci. 37, 479–505 (2009).
Sleep, N. H. & Zahnle, K. Carbon dioxide cycling and implications for climate on ancient Earth. J. Geophys. Res. 106, 1373–1399 (2001).
Kelley, D. S. et al. A serpentinite-hosted ecosystem: the Lost City Hydrothermal Field. Science 307, 1428–1434 (2005).
Proskurowski, G. et al. Abiogenic hydrocarbon production at Lost City hydrothermal field. Science 319, 604–607 (2008).
Klein, F., Grozeva, N. G. & Seewald, J. S. Abiotic methane synthesis and serpentinization in olivine-hosted fluid inclusions. Proc. Natl Acad. Sci. 116, 17666–17672 (2019).
Raymond, S. N., Schlichting, H. E., Hersant, F. & Selsis, F. Dynamical and collisional constraints on a stochastic late veneer on the terrestrial planets. Icarus 226, 671–681 (2013).
Krissansen-Totton, J., Arney, G. N. & Catling, D. C. Constraining the climate and ocean pH of the early Earth with a geological carbon cycle model. Proc. Natl Acad. Sci. 115, 4105–4110 (2018).
Elkins-Tanton, L. T. Linked magma ocean solidification and atmospheric growth for Earth and Mars. Earth Planet. Sci. Lett. 271, 181–191 (2008).
Lebrun, T. et al. Thermal evolution of an early magma ocean in interaction with the atmosphere. J. Geophys. Res. Planet. 118, 1155–1176 (2013).
Hamano, K., Abe, Y. & Genda, H. Emergence of two types of terrestrial planet on solidification of magma ocean. Nature 497, 607–610 (2013).
Salvador, A. et al. The relative influence of H2O and CO2 on the primitive surface conditions and evolution of rocky planets. J. Geophys. Res. Planet. 122, 1458–1486 (2017).
Bower, D. J. et al. Linking the evolution of terrestrial interiors and an early outgassed atmosphere to astrophysical observations. Astron. Astrophys. 631, A103 (2019).
Hirschmann, M. M. Magma ocean influence on early atmosphere mass and composition. Earth Planet. Sci. Lett. 341–344, 48–57 (2012).
Deng, J., Du, Z., Karki, B. B., Ghosh, D. B. & Lee, K. K. A magma ocean origin to divergent redox evolutions of rocky planetary bodies and early atmospheres. Nat. Commun. 11, 2007 (2020).
Abe, Y. Physical state of the very early Earth. Lithos 30, 223–235 (1993).
Catling, D. C. & Zahnle, K. J. The Archean atmosphere. Sci. Adv. 6, eaax1420 (2020).
Solomatov, V. S. In Treatise on Geophysics. Volume 9: Evolution of the Earth 1st edn (ed. Schubert G.) 91–119 (Elsevier, 2007).
Hier-Majumder, S. & Hirschmann, M. M. The origin of volatiles in the Earth’s mantle. Geochem. Geophys. Geosyst. 18, 3078–3092 (2017).
Kawamoto, T. & Holloway, J. R. Melting temperature and partial melt chemistry to H2O-saturated mantle peridotite to 11 gigapascals. Science 276, 240–243 (1997).
Katz, R. F., Spiegelman, M. & Langmuir, C. H. A new parameterization of hydrous mantle melting. Geochem. Geophys. Geosyst. 4, 1073 (2003).
Hirschmann, M. M. & Dasgupta, R. The H/C ratios of Earth’s near-surface and deep reservoirs, and consequences for deep Earth volatile cycles. Chem. Geol. 262, 4–16 (2009).
Korenaga, J., Planavsky, N. J. & Evans, D. A. D. Global water cycle and the coevolution of the Earth’s interior and surface environment. Philos. Trans. R. Soc. A 375, 20150393 (2017).
Maurice, M. et al. Onset of solid-state mantle convection and mixing during magma ocean solidification. J. Geophys. Res. Planet. 122, 577–598 (2017).
Miyazaki, Y. & Korenaga, J. On the timescale of magma ocean solidification and its chemical consequences: 2. Compositional differentiation under crystal accumulation and matrix compaction. J. Geophys. Res. Solid Earth 124, 3399–3419 (2019).
Blank, J. G. & Brooker, R. A. In Reviews in Mineralogy and Geochemistry. Volume 30: Volatiles in Magmas (eds Carrol, M. R. & Holloway, J. R.) 157–186 (Mineralogical Society of America, 1994).
Abe, Y. In Evolution of the Earth and Planets (eds Takahashi, E. et al.) 41–54 (AGU, 1993).
Hirth, G. & Kohlstedt, D. L. Water in the oceanic upper mantle: implications for rheology, melt extraction and the evolution of the lithosphere. Earth Planet. Sci. Lett. 144, 93–108 (1996).
Jain, C., Korenaga, J. & Karato, S.-i Global analysis of experimental data on the rheology of olivine aggregates. J. Geophys. Res. Solid Earth 124, 310–334 (2019).
Korenaga, J. Thermal evolution with a hydrating mantle and the initiation of plate tectonics in the early Earth. J. Geophys. Res. 116, B12403 (2011).
Korenaga, J. Plate tectonics and surface environment: role of the oceanic upper mantle. Earth Sci. Rev. 205, 103185 (2020).
Zahnle, K. et al. Emergence of a habitable planet. Space Sci. Rev. 129, 35–78 (2007).
Korenaga, J. Energetics of mantle convection and the fate of fossil heat. Geophys. Res. Lett. 30, 1437 (2003).
Bradley, D. C. Passive margins through earth history. Earth Sci. Rev. 91, 1–26 (2008).
Herzberg, C., Condie, K. & Korenaga, J. Thermal history of the Earth and its petrological expression. Earth Planet. Sci. Lett. 292, 79–88 (2010).
Pehrsson, S. J., Eglington, B. M., Evans, D. A., Huston, D. & Reddy, S. M. Metallogeny and its link to orogenic style during the Nuna supercontinent cycle. Geol. Soc. Spec. Publ. 424, 83–94 (2016).
Plesa, A.-C., Tosi, N. & Breuer, D. Can a fractionally crystallized magma ocean explain the thermo-chemical evolution of Mars? Earth Planet. Sci. Lett. 403, 225–235 (2014).
Ghiorso, M. S., Hirschmann, M. M., Reiners, P. W. & Kress, V. C. III The pMELTS: A revision of MELTS for improved calculation of phase relations and major element partitioning related to partial melting of the mantle to 3 GPa. Geochem. Geophys. Geosyst. 3, 1–35 (2002).
Gualda, G. A., Ghiorso, M. S., Lemons, R. V. & Carley, T. L. Rhyolite-MELTS: a modified calibration of MELTS optimized for silica-rich, fluid-bearing magmatic systems. J. Petrol. 53, 875–890 (2012).
Korenaga, J. In Archean Geodynamics and Environments (eds Benn, K. et al.) 7–32 (AGU, 2006).
Davies, G. F. On the emergence of plate tectonics. Geology 20, 963–966 (1992).
Korenaga, J. Scaling of plate tectonic convection with pseudoplastic rheology. J. Geophys. Res. 115, B11405 (2010).
Diamond, L. W. & Akinfiev, N. N. Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: evaluation of literature data and thermodynamic modelling. Fluid Phase Equilib. 208, 265–290 (2003).
Alt, J. C. & Teagle, D. A. The uptake of carbon during alteration of ocean crust. Geochim. Cosmochim. Acta 63, 1527–1535 (1999).
Sleep, N. H., Meibom, A., Fridriksson, T., Coleman, R. G. & Bird, D. K. H2-rich fluids from serpentinization: geochemical and biotic implications. Proc. Natl Acad. Sci. 101, 12818–12823 (2004).
Schulte, M., Blake, D., Hoehler, T. & McCollom, T. Serpentinization and its implications for life on the early Earth and Mars. Astrobiology 6, 364–376 (2006).
Lambert, J. B., Gurusamy-Thangavelu, S. A. & Ma, K. The silicate-mediated formose reaction: bottom-up synthesis of sugar silicates. Science 327, 984–986 (2010).
Davies, G. F. Gravitational depletion of the early Earth’s upper mantle and the viability of early plate tectonics. Earth Planet. Sci. Lett. 243, 376–382 (2006).
Zahnle, K. J., Kasting, J. F. & Pollack, J. B. Evolution of a steam atmosphere during Earth’s accretion. Icarus 74, 62–97 (1988).
Dullien, F. A. L. Porous Media: Fluid Transport and Pore Structure 2nd edn (Academic, 1992).
Zahnle, K. J., Lupu, R., Dobrovolskis, A. & Sleep, N. H. The tethered Moon. Earth Planet. Sci. Lett. 427, 74–82 (2015).
Trønnes, R. G. & Frost, D. J. Peridotite melting and mineral-melt partitioning of major and minor elements at 22–24.5 GPa. Earth Planet. Sci. Lett. 197, 117–131 (2002).
Corgne, A., Liebske, C., Wood, B. J., Rubie, D. C. & Frost, D. J. Silicate perovskite-melt partitioning of trace elements and geochemical signature of a deep perovskitic reservoir. Geochim. Cosmochim. Acta 69, 485–496 (2005).
Parsons, B. Causes and consequences of the relation between area and age of the ocean floor. J. Geophys. Res. 87, 289–302 (1982).
Zhang, G., Mei, S. & Song, M. Effect of water on the dislocation creep of enstatite aggregates at 300 MPa. Geophys. Res. Lett. 47, e2019GL085895 (2020).
Aubaud, C., Hauri, E. H. & Hirschmann, M. M. Hydrogen partition coefficients between nominally anhydrous minerals and basaltic melts. Geophys. Res. Lett. 31, L20611 (2004).
de Capitani, C. & Petrakakis, K. The computation of equilibrium assemblage diagrams with Theriak/Domino software. Am. Mineral. 95, 1006–1016 (2010).
McKenzie, D. The generation and compaction of partially molten rock. J. Petrol. 25, 713–765 (1984).
Christensen, U. R. Thermal evolution models for the Earth. J. Geophys. Res. 90, 2995–3007 (1985).
Korenaga, J. Thermal cracking and the deep hydration of oceanic lithosphere: A key to the generation of plate tectonics? J. Geophys. Res. 112, B05408 (2007).
Tackley, P. J. In Treatise on Geophysics: Volume 7: Mantle Dynamics 2nd edn (ed. Schubert G.) 521–585 (Elsevier, 2015).
Nakajima, S., Hayashi, Y.-Y. & Abe, Y. A study on the “runaway greenhouse effect” with a one-dimensional radiative–convective equilibrium model. J. Atmos. Sci. 49, 2256–2266 (1992).
Johnson, S. S., Mischna, M. A., Grove, T. L. & Zuber, M. T. Sulfur-induced greenhouse warming on early Mars. J. Geophys. Res. Planet. 113, E08005 (2008).
Abe, Y. & Matsui, T. The formation of an impact-generated H2O atmosphere and its implications for the early thermal history of the Earth. J. Geophys. Res. Suppl. 90, C545–C559 (1985).
Dasgupta, R. & Hirschmann, M. M. The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett. 298, 1–13 (2010).
Kelemen, P. B. & Manning, C. E. Reevaluating carbon fluxes in subduction zones, what goes down, mostly comes up. Proc. Natl Acad. Sci. 112, E3997–E4006 (2015).
Sleep, N. H., Zahnle, K. & Neuhoff, P. S. Initiation of clement surface conditions on the earliest Earth. Proc. Natl Acad. Sci. 98, 3666–3672 (2001).
Peterson, M. N. A. Calcite: rates of dissolution in a vertical profile in the central Pacific. Science 154, 1542–1544 (1966).
Andersson, A. J. In Treatise on Geochemistry. Volume 8: The Oceans and Marine Geochemistry 2nd edn (eds Holland, H. D. & Turekian, K.) 519–542 (Elsevier, 2014).
Kelemen, P. B. et al. Rates and mechanisms of mineral carbonation in peridotite: natural processes and recipes for enhanced, in situ CO2 capture and storage. Annu. Rev. Earth Planet. Sci. 39, 545–576 (2011).
Syracuse, E. M., van Keken, P. E. & Abers, G. A. The global range of subduction zone thermal models. Phys. Earth Planet. Inter. 183, 73–90 (2010).
Dasgupta, R., Hirschmann, M. M. & Withers, A. C. Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth Planet. Sci. Lett. 227, 73–85 (2004).
Korenaga, J. On the extent of mantle hydration caused by plate bending. Earth Planet. Sci. Lett. 457, 1–9 (2017).
Miller, N. C., Lizarralde, D., Collins, J. A., Holbrook, W. S. & Van Avendonk, H. J. Limited mantle hydration by bending faults at the middle America trench. J. Geophys. Res. Solid Earth 126, e2020JB020982 (2021).
Miyazaki, Y. & Korenaga, J. Effects of chemistry on vertical dust motion in early protoplanetary disks. Astrophys. J. 849, 41 (2017).
Wirth, E. A. & Korenaga, J. Small-scale convection in the subduction zone mantle wedge. Earth Planet. Sci. Lett. 357–358, 111–118 (2012).
Lyubetskaya, T. & Korenaga, J. Chemical composition of Earth’s primitive mantle and its variance: 1. Method and results. J. Geophys. Res. 112, B03211 (2007).
Gale, A., Dalton, C. A., Langmuir, C. H., Su, Y. & Schilling, J.-G. The mean composition of ocean ridge basalts. Geochem. Geophys. Geosyst. 14, 489–518 (2013).
Acknowledgements
This work was sponsored by the US National Aeronautics and Space Administration under Cooperative Agreement No. 80NSSC19M0069 issued through the Science Mission Directorate and the National Science Foundation under grant EAR-1753916. This work was also supported in part by the facilities and staff of the Yale University Faculty of Arts and Sciences High Performance Computing Center. Y.M. was supported by the Stanback Postdoctoral Fellowship from Caltech Center for Comparative Planetary Evolution. The authors thank N. Sleep and M. Hirschmann for providing constructive comments, which were helpful to substantially improve the accuracy of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.M. and J.K. designed the study, discussed the results, and wrote the manuscript. Y.M. performed calculations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Peer review
Peer review information
Nature thanks Marc Hirschmann and Norman Sleep for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The thermal structure of the chemically heterogeneous mantle plotted together with contours of melt fraction.
a–c, Melt fraction is shown for two components of the mantle: high-Mg# pyroxenite matrix (a) and Fe-rich blobs (c), and water content remaining in the solid phase of the high-Mg# matrix (b). The melt fractions of high-Mg# matrix and Fe-rich blobs are estimated using the Rhyolite-MELTS model42. The dry (grey solid) and wet (0.1 wt%; grey dashed) adiabats are calculated with a potential temperature of 1,600 °C, using equation (17). For the wet adiabat of the high-Mg# matrix, two modes of melting are considered, batch (dotted) and fractional (dashed), and for the latter, we assume that 90% of melt would escape from the mantle for every 0.1 GPa of ascent when the degree of melting is greater than 1%. The fraction of melt escaping the system is insensitive to the thickness of complete dehydration, and values between 10% and 99.9% would yield a thickness within 0.1 GPa of what is predicted in this figure. d, Schematic illustration of the compositional structure of the mantle during and after the solidification of a magma ocean under fractional crystallization. The mantle experiences global-scale chemical stratification, leaving an Fe-rich layer near the surface. Such stratification is subject to the Rayleigh–Taylor (RT) instability, resulting in the dripping-like descent of Fe-rich materials. This period corresponds to Fig. 1a. These droplets would solidify as they sink through the mantle (left) and be mixed with high-Mg# cumulates. When the surface becomes rheologically solid (Fig. 1b), the mantle would have a structure with small-scale chemical heterogeneity, embedded in high-Mg# matrix (right).
Extended Data Fig. 2 Thermal structure of a pyrolitic (chemically homogeneous) mantle when a melt-dominated layer disappears.
The figure describes when the entire mantle starts to behave rheologically as solid, which corresponds to a potential temperature of ~1,600 °C. a–c, The profiles of temperature (a), melt fraction (b) and water content (c) are shown for both dry (grey solid) and wet (0.1 wt%; grey dotted and dashed) mantles, together with the solidus (blue) and liquidus of pyrolite (red). The solidus of a wet mantle (blue dashed) is estimated using equation (18) and a melt/mineral partitioning coefficient of D = 0.005. Two modes of melting are considered for the wet mantle, batch (dotted) and fractional (dashed). Temperature profiles would be adiabatic as a result of the Rayleigh–Taylor instability, and melt fraction is estimated using a model of ref. 24 with the mass fraction of clinopyroxene of 19 wt%. For fractional melting, we assume that 90% of melt would escape from the mantle for every 0.1 GPa of ascent when the degree of melting is greater than 1%.
Extended Data Fig. 3 The subduction geotherm during the Hadean with the stability field of carbonates.
P–T paths are calculated assuming a dip angle of 45° using the model of ref. 79 (see Methods for details). We consider (1) a mantle potential temperature of 1,350 °C with a plate age of 10 Myr, a velocity of 5 cm yr−1, and a surface temperature of 0 °C (blue), (2) a potential temperature of 1,600 °C with a plate age of 10 Myr, a velocity of 50 cm yr−1, and a surface temperature of 230 °C (red solid), and (3) a potential temperature of 1,600 °C with a plate age of 100 Myr, a velocity of 5 cm yr−1, and a surface temperature of 230 °C (red dashed), representing the present-day young slab, the Hadean slab of the chemically heterogeneous mantle, and that of a homogeneous pyrolitic mantle, respectively. P–T paths of the heterogeneous and homogenous mantles are similar because rapid plate motion mitigates the heating of the slab from the surrounding mantle. The solidus of carbonate melt is adopted from ref. 75 (black dotted), and the decomposition of magnesite under the presence of quartz is calculated using Theriak-Domino60 (grey dotted).
Extended Data Fig. 4 The profiles of Al2O3 (red) and CaO (blue) contents after magma ocean solidification.
Profiles before the onset of small-scale Rayleigh–Taylor instabilities are shown. The top 500 km of the mantle becomes the source for iron-rich blobs, whereas the lower mantle composition corresponds to the high-Mg# matrix in the main text. We assume that newly formed crystals in the magma ocean would stack at the base of the melt-dominated layer (Fig. 1a). Initial concentrations of Al2O3 3.5 wt% and CaO 2.8 wt%80 are assumed (dashed lines), and partitioning coefficients between melt and bridgmanite are calculated from the experimental results of Trønnes and Frost55 and Corgne et al56. Shaded areas represent uncertainties, which are calculated using partitioning coefficients 30% larger and smaller values than the mean estimated values (DAl/Si = 0.78 and DCa/Si = 0.16).
Extended Data Fig. 5 Effective viscosity contrast across the lithosphere and the criteria for plate tectonics.
Effective viscosity contrast is shown (equation (14)) as a function of the internal Rayleigh number, together with the corresponding mantle potential temperature. The thickness of depleted lithospheric mantle hm in equation (14) is different between chemically homogeneous (grey) and heterogeneous mantles (red), and the values of hm are shown in Fig. 3b. The criteria for plate tectonics (ΔηL,crit in equation (20)) is also plotted with a dotted line.
Supplementary information
Rights and permissions
About this article
Cite this article
Miyazaki, Y., Korenaga, J. A wet heterogeneous mantle creates a habitable world in the Hadean. Nature 603, 86–90 (2022). https://doi.org/10.1038/s41586-021-04371-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04371-9
This article is cited by
-
Magnesium silicate chimneys at the Strytan hydrothermal field, Iceland, as analogues for prebiotic chemistry at alkaline submarine hydrothermal vents on the early Earth
Progress in Earth and Planetary Science (2024)
-
The importance of continents, oceans and plate tectonics for the evolution of complex life: implications for finding extraterrestrial civilizations
Scientific Reports (2024)
-
Hadean mantle oxidation inferred from melting of peridotite under lower-mantle conditions
Nature Geoscience (2023)
-
Long-lived volcanic resurfacing of Venus driven by early collisions
Nature Astronomy (2023)
-
Temperature dependence of nitrogen solubility in bridgmanite and evolution of nitrogen storage capacity in the lower mantle
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.