Abstract
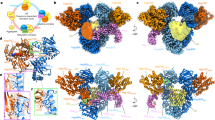
Hsp90 is a conserved and essential molecular chaperone responsible for the folding and activation of hundreds of ‘client’ proteins1,2,3. The glucocorticoid receptor (GR) is a model client that constantly depends on Hsp90 for activity4,5,6,7,8,9. GR ligand binding was previously shown to be inhibited by Hsp70 and restored by Hsp90, aided by the co-chaperone p2310. However, a molecular understanding of the chaperone-mediated remodelling that occurs between the inactive Hsp70–Hsp90 ‘client-loading complex’ and an activated Hsp90–p23 ‘client-maturation complex’ is lacking for any client, including GR. Here we present a cryo-electron microscopy (cryo-EM) structure of the human GR-maturation complex (GR–Hsp90–p23), revealing that the GR ligand-binding domain is restored to a folded, ligand-bound conformation, while being simultaneously threaded through the Hsp90 lumen. In addition, p23 directly stabilizes native GR using a C-terminal helix, resulting in enhanced ligand binding. This structure of a client bound to Hsp90 in a native conformation contrasts sharply with the unfolded kinase–Hsp90 structure11. Thus, aided by direct co-chaperone–client interactions, Hsp90 can directly dictate client-specific folding outcomes. Together with the GR-loading complex structure12, we present the molecular mechanism of chaperone-mediated GR remodelling, establishing the first, to our knowledge, complete chaperone cycle for any Hsp90 client.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The cryo-EM maps generated in this study have been deposited in the Electron Microscopy Data Bank (EMDB) under the accession codes EMD-23004 (GR–Hsp90–p23), EMD-23006 (Hsp90–p23) and EMD-23005 (MBP–Hsp90–p23). The atomic coordinates have been deposited in the PDB under the accession code 7KRJ (GR–Hsp90–p23). Publicly available PDB entries used in this study are: 5FWK, 1M2Z, 4P6X, 1EJF, 2CG9, 1OMP and 1ANF. The human p23 structure prediction is available from AlphaFold v2.0 with the accession code P83868. Source data are provided with this paper.
References
Taipale, M., Jarosz, D. F. & Lindquist, S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515–528 (2010).
Schopf, F. H., Biebl, M. M. & Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 18, 345–360 (2017).
Taipale, M. et al. Quantitative analysis of HSP90–client interactions reveals principles of substrate recognition. Cell 150, 987–1001 (2012).
Picard, D. et al. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 348, 166–168 (1990).
Pratt, W. B. & Toft, D. O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocrine Rev. 18, 306–360 (1997).
Morishima, Y., Murphy, P. J., Li, D. P., Sanchez, E. R. & Pratt, W. B. Stepwise assembly of a glucocorticoid receptor·hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J. Biol. Chem. 275, 18054–18060 (2000).
Smith, D. F. & Toft, D. O. Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol. Endocrinol. 22, 2229–2240 (2008).
Lorenz, O. R. et al. Modulation of the Hsp90 chaperone cycle by a stringent client protein. 53, 941–953 (2014).
Nathan, D. F. & Lindquist, S. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol. Cell. Biol. 15, 3917–3925 (1995).
Kirschke, E., Goswami, D., Southworth, D., Griffin, P. & Agard, D. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 157, 1685–1697 (2014).
Verba, K. A. et al. Atomic structure of Hsp90–Cdc37–Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 352, 1542–1547 (2016).
Wang, R. Y.-R. et al. Structure of Hsp90–Hsp70–Hop–GR reveals the Hsp90 client-loading mechanism. Nature https://doi.org/10.1038/s41586-021-04252-1 (2021).
Zhao, R. et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120, 715–727 (2005).
Rosenzweig, R., Nillegoda, N. B., Mayer, M. P. & Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 20, 665–680 (2019).
Krukenberg, K. A., Street, T. O., Lavery, L. A. & Agard, D. A. Conformational dynamics of the molecular chaperone Hsp90. Q. Rev. Biophys. 44, 229–255 (2011).
Ali, M. M. U. et al. Crystal structure of an Hsp90–nucleotide–p23/Sba1 closed chaperone complex. Nature 440, 1013–1017 (2006).
Sahasrabudhe, P., Rohrberg, J., Biebl, M. M., Rutz, D. A. & Buchner, J. The plasticity of the Hsp90 co-chaperone system. Mol. Cell 67, 947–961.e945 (2017).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Wang, R. Y. et al. Automated structure refinement of macromolecular assemblies from cryo-EM maps using Rosetta. eLife 5, e17219 (2016).
Meyer, P. et al. Structural and functional analysis of the middle segment of Hsp90: implications for ATP hydrolysis and client protein and cochaperone interactions. Mol. Cell 11, 647–658 (2003).
Rutz, D. A. et al. A switch point in the molecular chaperone Hsp90 responding to client interaction. Nat. Commun. 9, 1472 (2018).
Hawle, P. et al. The middle domain of Hsp90 acts as a discriminator between different types of client proteins. Mol. Cell. Biol. 26, 8385–8395 (2006).
Bledsoe, R. K. et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. 110, 93–105 (2002).
Weikl, T., Abelmann, K. & Buchner, J. An unstructured C-terminal region of the Hsp90 co-chaperone p23 is important for its chaperone function. J. Mol. Biol. 293, 685–691 (1999).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Seraphim, T. V. et al. The C-terminal region of the human p23 chaperone modulates its structure and function. Arch. Biochem. Biophys. 565, 57–67 (2015).
Biebl, M. M. et al. Structural elements in the flexible tail of the co-chaperone p23 coordinate client binding and progression of the Hsp90 chaperone cycle. Nat. Commun. 12, 828 (2021).
de Castro, E. et al. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 34, W362–W365 (2006).
McKenna, N. J. & O’Malley, B. W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108, 465–474 (2002).
Weaver, A. J., Sullivan, W. P., Felts, S. J., Owen, B. A. L. & Toft, D. O. Crystal structure and activity of human p23, a heat shock protein 90 co-chaperone. J. Biol. Chem. 275, 23045–23052 (2000).
Freeman, B. C., Toft, D. O. & Morimoto, R. I. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science 274, 1718–1720 (1996).
Freeman, B. C., Felts, S. J., Toft, D. O. & Yamamoto, K. R. The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev. 14, 422–434 (2000).
Bohen, S. P. Genetic and biochemical analysis of p23 and ansamycin antibiotics in the function of Hsp90-dependent signaling proteins. Mol. Cell. Biol. 18, 3330–3339 (1998).
Freeman, B. C. & Yamamoto, K. R. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296, 2232–2235 (2002).
Liu, Y., Elnatan, D., Sun, M., Myasnikov, A. G. & Agard, D. A. Cryo-EM reveals the dynamic interplay between mitochondrial Hsp90 and SdhB folding intermediates. Preprint at https://doi.org/10.1101/2020.10.06.327627 (2020).
Suren, T. et al. Single-molecule force spectroscopy reveals folding steps associated with hormone binding and activation of the glucocorticoid receptor. Proc. Natl Acad. Sci. USA 115, 11688–11693 (2018).
Czar, M. J., Galigniana, M. D., Silverstein, A. M. & Pratt, W. B. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry 36, 7776–7785 (1997).
Galigniana, M. D., Radanyi, C., Renoir, J. M., Housley, P. R. & Pratt, W. B. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J. Biol. Chem. 276, 14884–14889 (2001).
Netzer, W. J. & Hartl, F. U. Recombination of protein domains facilitated by co-translational folding in eukaryotes. Nature 388, 343–349 (1997).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Johnson, J. L. & Toft, D. O. Binding of p23 and hsp90 during assembly with the progesterone receptor. Mol. Endocrinol. 9, 670–678 (1995).
Csermely, P. et al. Atp induces a conformational change of the 90-Kda heat-shock protein (Hsp90). J. Biol. Chem. 268, 1901–1907 (1993).
Schorb, M., Haberbosch, I., Hagen, W. J. H., Schwab, Y. & Mastronarde, D. N. Software tools for automated transmission electron microscopy. Nat. Methods 16, 471–477 (2019).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zimmermann, L. et al. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 430, 2237–2243 (2018).
Song, Y. et al. High-resolution comparative modeling with RosettaCM. Structure 21, 1735–1742 (2013).
Sharff, A. J., Rodseth, L. E., Spurlino, J. C. & Quiocho, F. A. Crystallographic evidence of a large ligand-induced hinge-twist motion between the two domains of the maltodextrin binding protein involved in active transport and chemotaxis. Biochemistry 31, 10657–10663 (1992).
Quiocho, F. A., Spurlino, J. C. & Rodseth, L. E. Extensive features of tight oligosaccharide binding revealed in high-resolution structures of the maltodextrin transport/chemosensory receptor. Structure 5, 997–1015 (1997).
UniProt, C. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489 (2021).
Madeira, F. et al. The EMBL–EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47, W636–W641 (2019).
Waterhouse, A. M., Procter, J. B., Martin, D. M., Clamp, M. & Barton, G. J. Jalview version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Jones, D. T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292, 195–202 (1999).
Landau, M. et al. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 33, W299–W302 (2005).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
He, Y. et al. Structures and mechanism for the design of highly potent glucocorticoids. Cell Res. 24, 713–726 (2014).
Pei, J. & Grishin, N. V. AL2CO: calculation of positional conservation in a protein sequence alignment. Bioinformatics 17, 700–712 (2001).
Mirabello, C. & Pollastri, G. Porter, PaleAle 4.0: high-accuracy prediction of protein secondary structure and relative solvent accessibility. Bioinformatics 29, 2056–2058 (2013).
Kallberg, M. et al. Template-based protein structure modeling using the RaptorX web server. Nat. Protoc. 7, 1511–1522 (2012).
Johnson, J. L., Halas, A. & Flom, G. Nucleotide-dependent interaction of Saccharomyces cerevisiae Hsp90 with the cochaperone proteins Sti1, Cpr6, and Sba1. Mol. Cell. Biol. 27, 768–776 (2007).
Johnson, J. L. & Craig, E. A. A role for the Hsp40 Ydj1 in repression of basal steroid receptor activity in yeast. Mol. Cell. Biol. 20, 3027–3036 (2000).
Acknowledgements
We thank members of the Agard laboratory and E. Kirschke for helpful discussions; D. Bulkley, G. Gilbert, Z. Yu and E. Tse from the W. M. Keck Foundation Advanced Microscopy Laboratory at the University of California, San Francisco (UCSF) for EM facility maintenance and help with data collection; and M. Harrington and J. Baker-LePain for computational support with the UCSF Wynton cluster. C.M.N. is a National Cancer Institute Ruth L. Kirschstein Predoctoral Individual NRSA Fellow. R.Y.-R.W. was a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation. The work was supported by funding from Howard Hughes Medical Institute (D.A.A.) and NIH grants R35GM118099 (D.A.A.), S10OD020054 (D.A.A.), S10OD021741 (D.A.A.), P20GM104420 (J.L.J.) and R01GM127675 (J.L.J.).
Author information
Authors and Affiliations
Contributions
C.M.N. and R.Y.-R.W. designed and executed biochemical experiments, cryo-EM sample preparation, data collection, data processing and model building. J.L.J. executed yeast in vivo assays and interpreted the results. C.M.N., R.Y.-R.W. and D.A.A. conceived the project, interpreted the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature thanks Matthias Mayer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Sample Preparation.
a, Coomassie-stained raw, uncropped SDS-PAGE (4-12% acrylamide gel) with elution from the MBP-GR pulldown from the in vitro reconstituted GR chaperone cycle. Assay conditions are as follows- Lane 1: 5 μM MBP-GR only; Lane 2-5: 5 μM MBP-GR, 2 μM Hsp40, 5 μM Hsp70, 5 μM Hop, 15 μM Hsp90, 15 μM Bag-1, 30 μM p23, 5 mM ATP. Lane 2: no Molybdate addition, Lane 3: addition of 20mM Molybdate, Lane 4: addition of 20 mM Molybdate after 1 h pre-incubation (see Methods) b, Shodex KW-804 size exclusion chromatography profile of the GR-maturation complex purified by MBP-GR pulldown from the reconstituted GR chaperone cycle. mAU=milli-absorbance units. c, Coomassie-stained raw, uncropped SDS-PAGE (4-12% acrylamide gel) of the fractions from size exclusion chromatography. Colors indicate which gel lanes correspond to specific regions of the size exclusion chromatography profile. Sample fractions from the region highlighted in purple were collected and used for cryo-EM data collection. This experiment was repeated 4 independent times with similar results. d, Representative electron micrograph for the cryo-EM dataset (−2.32 μm defocus). A total of 5608 micrographs were obtained. Scale bar is 20nm. e, Domain organization of the proteins in the GR-maturation complex.
Extended Data Fig. 2 Cryo-EM Data Analysis.
a, Cryo-EM data processing procedure with 3D reconstructions colored by local resolution. b, Gold-standard Fourier shell correlation (FSC) curves of the 3D reconstructions. The black dashed lines intercept the y-axis at an FSC value of 0.143. For the GR:Hsp90:p23 reconstruction, the map-model FSC is plotted in blue, with the blue dashed line intercepting the y-axis at an FSC value of 0.5. c, GR maturation complex map density with atomic model showing ATP-magnesium density in both Hsp90 protomers (Hsp90A/B). Bottom images show increased contour level on the map density to indicate that the ATP γ-phosphate position has much stronger density relative to the α and β-phosphates, likely corresponding to molybdate, which may act as a γ-phosphate analog (see Methods).
Extended Data Fig. 3 Hsp90:GR Interfaces.
Atomic model of the maturation complex with Hsp90A (dark blue), Hsp90B (light blue), GR (yellow). a, View of the GRpre-helix 1 strand threaded through the Hsp90 lumen and GR helices 1 and 3 packing against the entrance to the Hsp90 lumen. Side chains on GR in contact with Hsp90 are shown. Hsp90A/B are in surface representation. Hydrophobic residues on Hsp90 are colored in pink. b, Interface 1 of the Hsp90:GR interaction depicting the GRpre-Helix 1 region (GR523–531) threading through the Hsp90 lumen. Side chains in contact between GR and Hsp90 are shown, along with hydrogen bonds (dashed pink lines). c, Interface 2 of the Hsp90:GR interaction depicting GRHelix 1 (GR532–539) packing against Hsp90B. Side chains in contact between GR and Hsp90 are shown, along with hydrogen bonds (dashed pink lines). d, Interface 3 of the Hsp90:GR interaction depicting residues on the Hsp90AMD loops (Hsp90AN318,W320,F349,R346) and Hsp90Bamphi-α (Hsp90BT624,Y627,M628) packing against GR. Side chains in contact between GR and Hsp90 are shown, along with hydrogen bonds (dashed pink lines).
Extended Data Fig. 4 GR is in a Native, Ligand-Bound State in the Maturation Complex.
a, Atomic model of GR from the maturation complex (yellow) compared with GR from the crystal structure (PDB ID 1M2Z) (light pink) with co-activator peptide NCoA2 (purple) and ligand (pink). GRHelix 12 is indicated. b, GR-maturation complex atomic model in surface representation with the co-activator peptide NCoA2 (purple) docked based on the GR:NCoA2 crystal structure (PDB ID M2Z). The NCoA2 peptide binding interface is available and the bound NCoA2 peptide does not clash with Hsp90. Hsp90A (dark blue), Hsp90B (light blue), GR (yellow), p23 (green). c, GR maturation complex map density (sharpened with B factor −40) with either the dexamethasone-bound crystal structure docked (left panel, PDB ID 1M2Z) or the cortisol-bound crystal structure docked (right panel, PDB ID 4P6X) into the GR map density. In the top images, the ligand density is shown with the agonist dexamethasone (left) or the agonist cortisol (right) from the docked crystal structures. Arrow indicates the extra carbon atom in dexamethasone compared to cortisol. In the bottom images, density for GRY735 is shown with either the dexamethasone-bound crystal structure docked (left) or the cortisol-bound crystal structure docked (right). d, GR-maturation complex atomic model in surface representation depicting the GR LBD dimerization interface. Hsp90A (dark blue), Hsp90B (light blue), GR (yellow), p23 (green). Left, the GR LBD dimerization interface is highlighted (light pink). Right, while the dimerization interface is solvent accessible in the GR-maturation complex, the binding of a second GR LBD (light pink) clashes with the Hsp90B CTD. The dimerization interface is based on the GR LBD dimer crystal structure (PDB ID 1M2Z).
Extended Data Fig. 5 Hsp90:p23 Interfaces.
Atomic model of the maturation complex with Hsp90A (dark blue), Hsp90B (light blue), GR (yellow), p23 (green). b, Interface 1 of the Hsp90:p23 interaction depicting Hsp90B interacting with one side of the p23 core. Side chains in contact between p23 and Hsp90B are shown, along with hydrogen bonds (dashed pink lines). c, Interface 2 of the Hsp90:p23 interaction depicting Hsp90B interacting with the base of the p23 core. Side chains in contact between p23 and Hsp90B are shown, along with hydrogen bonds (dashed pink lines). d, Interface 3 of the Hsp90:p23 interaction depicting Hsp90A interacting with the side of the p23 core. Side chains in contact between p23 and Hsp90 are shown, along with hydrogen bonds (dashed pink lines). e, Atomic model of a symmetric Hsp90 dimer (orange) compared with Hsp90 from the maturation complex atomic model, indicating a slight asymmetry in the Hsp90 dimer interface in the maturation complex. Hsp90A (dark blue), Hsp90B (light blue), p23 (green).
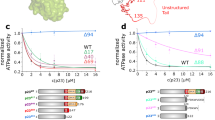
Extended Data Fig. 6 The p23tail helix:GR Interface.
a, Focused map of GR:p23tail helix showing density for the p23 tail with the atomic model built in. GR (yellow), p23 (green). b, Interface between the p23tail helix (green) and GR (colored by hydrophobicity, surface representation) showing that the p23tail helix binds to a hydrophobic patch on GR. p23 side chains interacting with GR are shown. c, Sequence identity across human SHRs (GR, mineralocorticoid receptor, androgen receptor, progesterone receptor, estrogen receptor α and β) plotted onto the GR structure. The p23tail helix (light green) was overlaid to indicate the p23:GR interface. d, Secondary structure predictions for human p23 from four different servers. Porter 4.0 (orange), RaptorX (blue), Psipred (purple), AlphaFold v2.0 (pink). The p23tail helix from the maturation complex atomic model is shown with the top green line. e, Atomic model of GR (yellow) and p23 (green) from the maturation complex highlighting the interaction between the p23tail helix and the GR C-terminus, which connects to GRHelix 12. f, Sequence alignment of eukaryotic p23 showing conservation of the p23tail helix sequence. The p23tail helix from the maturation complex atomic model is shown with the top green line. The bottom aligned sequence is the p23tail helix -like motif identified in NCoA3 using the ScanProsite server. Red boxes on the S. cerevisiae p23 sequence indicate predicted helices from the PsiPred server. The alignment is colored according to the ClustalW convention.
Extended Data Fig. 7 Effect of p23 Tail Mutants on GR Activity and Cell Survival.
a, Depiction of the two p23 tail mutants used in the GR activity assays. b, Individual data points corresponding to Fig. 2d. Equilibrium binding of 20 nM fluorescent dexamethasone to 250 nM GR with chaperones and p23 tail mutants measured by fluorescence polarization (mean±SD). n=3 biologically independent samples per condition (n=6 biologically independent samples for the GR only condition). Significance was evaluated using a one-way ANOVA (F(3,8) = 636.2; p < 0.0001) with post-hoc Dunnett’s multiple comparisons test (n.s. P ≥ 0.05; * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001). P-values: p(p23 vs. p23Δtail) = 0.1512, p(p23 vs. p23ΔhelixΔtail) = 0.0002, p(p23 vs. no p23) = <0.0001. c, Equilibrium binding of 20 nM fluorescent dexamethasone to 250 nM GR with addition of 15 μM p23 or p23 tail mutants measured by fluorescence polarization (mean±SD). n=7 biologically independent samples per condition (n=6 biologically independent samples for the GR + p23Δtail condition). Fluorescence polarization values are baseline subtracted in accordance with the measured fluorescent dexamethasone baseline polarization value. There were no statistically significant differences between group means as determined by a one-way ANOVA (F(3,23) = 1.708; p=0.1933). d, Yeast survival assay with human p23 or p23 tail mutants. Top panels: hsc82hsp82Δ yeast expressing Hsc82 I588A-M589A exhibit a growth defect at 37 °C in the presence of SBA1 and enhanced defects in cells lacking SBA1 (sba1). Bottom panels: Growth is restored by addition of human p23, although p23ΔhelixΔtail exhibits reproducibly reduced growth relative to p23 or p23Δtail. e, GR activation assay in wild-type yeast strain JJ762 expressing p23, p23 mutants, or Sba1 in addition to wild-type amounts of Sba1 from the native promoter. The fold increase in GR activities compared to the empty vector (e.v.) control are shown (mean±SD). n=18 biologically independent samples per condition (10 independent samples for the +Sba1 condition). Significance was evaluated using a one-way ANOVA (F(4,77) = 7.077; p < 0.0001) with post-hoc Šídák’s multiple comparisons test (n.s. P ≥ 0.05; * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001). P-values: p(e.v. vs. p23) = 0.0001, p(p23 vs p23Δtail) = 0.0164, p(p23 vs. p23ΔhelixΔtail) = 0.0021, p(p23 vs. Sba1) = 0.0002, p(p23Δtail vs. p23ΔhelixΔtail) = 0.9721. f, Expression of human p23 or p23 tail mutants in wild-type yeast strain JJ762 assayed by immunoblot with polyclonal antisera raised against Sba1 or human p23.
Extended Data Fig. 8 Hsp90:p23 Complex.
a, Cryo-EM density map of the Hsp90:p23 complex. Hsp90A (dark blue), Hsp90B (light blue), p23 (green). This color scheme is maintained in all figures that show the structure. b, Atomic model of Hsp90 and p23 from the GR-maturation complex docked into the Hsp90:p23 map density. c, Top view of the Hsp90:p23 complex density map with clipping plane to show unidentified density (gray) through the Hsp90 lumen. d, Cartoon representation of the Hsp90:p23 complex illustrating that MBP and GR LBD are not present in the map density (represented by a gray box).
Extended Data Fig. 9 MBP:Hsp90:p23 Complex.
a, Cryo-EM density map of the MBP:Hsp90:p23 complex. Far right image shows the density map lowpass-filtered to 8Å. Hsp90A (dark blue), Hsp90B (light blue), p23 (green), MBP (orange). This color scheme is maintained throughout b, Apo MBP crystal structure (PDB ID 1OMP) and atomic model of Hsp90 and p23 from the GR-maturation complex docked into the MBP:Hsp90:p23 map density. Note the missing density for the two MBP C-terminal helices. Far right image shows the density map lowpass-filtered to 8Å. c, Maltose-bound MBP crystal structure (PDB ID 1ANF) docked into the MBP:Hsp90:p23 map density. MBP (orange), maltose (pink). d, Top view of the MBP:Hsp90:p23 complex density map with clipping plane to show unidentified density (gray) through the Hsp90 lumen. e, Cartoon representation of the MBP:Hsp90:p23 complex illustrating the GR LBD is not present in the map density (represented by a gray box).
Extended Data Fig. 10 Comparison of the GR-Maturation Complex with the Hsp90:Kinase Complex.
a, Structure of Hsp90 bound to an unfolded kinase client (PDB ID 5FWK) with a strand of the kinase client threaded through the Hsp90 lumen. The two hydrophobic residues on the kinase (Cdk4V89,V92) that occupy the Hsp90 hydrophobic pockets are displayed. In the GR-maturation complex, two hydrophobic residues on GR (GRL525,L528) occupy the Hsp90 hydrophobic pockets, demonstrating a conserved client binding mode. Hsp90A (dark blue, surface representation), Hsp90B (light blue, surface representation), Cdk4 kinase (purple). Hydrophobic residues on Hsp90 are colored in pink. b, Structure of Hsp90 bound to an unfolded kinase client (PDB ID 5FWK) depicting Hsp90AF341 (Hsp90 isoform β) and Hsp90Bamphi-α packing against the kinase. In the GR-maturation complex, the corresponding residue Hsp90AF349 (Hsp90 isoform α) and the Hsp90Bamphi-α also pack against GR, demonstrating a conserved Hsp90:client binding interface. Hsp90A (dark blue), Hsp90B (light blue), Cdk4 kinase (purple, surface representation). c, Top, atomic models of the GR-maturation complex and Hsp90:kinase complex showing that both clients thread through the closed Hsp90 lumen. Hsp90A (dark blue, surface representation), Hsp90B (light blue, surface representation), GR (yellow), Cdk4 kinase (purple). Bottom, schematics demonstrating that both clients thread through the mostly hydrophobic Hsp90 lumen, but have different folding outcomes (H=hydrophobic interface).
Supplementary information
Rights and permissions
About this article
Cite this article
Noddings, C.M., Wang, R.YR., Johnson, J.L. et al. Structure of Hsp90–p23–GR reveals the Hsp90 client-remodelling mechanism. Nature 601, 465–469 (2022). https://doi.org/10.1038/s41586-021-04236-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04236-1
This article is cited by
-
Tracing genetic diversity captures the molecular basis of misfolding disease
Nature Communications (2024)
-
Dynamic stability of Sgt2 enables selective and privileged client handover in a chaperone triad
Nature Communications (2024)
-
Structural dynamics of RAF1-HSP90-CDC37 and HSP90 complexes reveal asymmetric client interactions and key structural elements
Communications Biology (2024)
-
Molecular Investigation and Preliminary Validation of Candidate Genes Associated with Neurological Damage in Heat Stroke
Molecular Neurobiology (2024)
-
Effects of the Glucocorticoid-Mediated Mitochondrial Translocation of Glucocorticoid Receptors on Oxidative Stress and Pyroptosis in BV-2 Microglia
Journal of Molecular Neuroscience (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.