Abstract
Replisome disassembly is the final step of eukaryotic DNA replication and is triggered by ubiquitylation of the CDC45–MCM–GINS (CMG) replicative helicase1,2,3. Despite being driven by evolutionarily diverse E3 ubiquitin ligases in different eukaryotes (SCFDia2 in budding yeast1, CUL2LRR1 in metazoa4,5,6,7), replisome disassembly is governed by a common regulatory principle, in which ubiquitylation of CMG is suppressed before replication termination, to prevent replication fork collapse. Recent evidence suggests that this suppression is mediated by replication fork DNA8,9,10. However, it is unknown how SCFDia2 and CUL2LRR1 discriminate terminated from elongating replisomes, to selectively ubiquitylate CMG only after termination. Here we used cryo-electron microscopy to solve high-resolution structures of budding yeast and human replisome–E3 ligase assemblies. Our structures show that the leucine-rich repeat domains of Dia2 and LRR1 are structurally distinct, but bind to a common site on CMG, including the MCM3 and MCM5 zinc-finger domains. The LRR–MCM interaction is essential for replisome disassembly and, crucially, is occluded by the excluded DNA strand at replication forks, establishing the structural basis for the suppression of CMG ubiquitylation before termination. Our results elucidate a conserved mechanism for the regulation of replisome disassembly in eukaryotes, and reveal a previously unanticipated role for DNA in preserving replisome integrity.
Similar content being viewed by others
Main
The eukaryotic replisome is assembled around the CMG helicase at replication origins during replication initiation. Once assembled, CMG remains stably associated with replication forks until two forks emanating from adjacent origins converge, or a single fork encounters the end of a linear chromosome or a template discontinuity, at which point replication terminates (Fig. 1a). Upon termination, the replisome is disassembled in two steps: first, CMG is ubiquitylated on its Mcm7 subunit by a cullin-RING E3 ubiquitin ligase (SCFDia2 in budding yeast, CUL2LRR1 in metazoa); second, ubiquitylated Mcm7 is unfolded by the Cdc48 ATPase (also known as p97 in higher eukaryotes), leading to disassembly of the replisome1,2,3,8,9,10. As there is no known mechanism for origin-independent CMG assembly in S phase, premature disassembly of CMG must be avoided, to prevent replication fork collapse and genome instability11. CMG translocates on the leading-strand template while excluding the lagging-strand template from its central channel12. It has been suggested that this ‘excluded’ DNA strand, which is lost upon termination (Fig. 1a), inhibits ubiquitylation of CMG at replication forks8,9,10. However, because there are currently no structures of terminated replisomes in complex with SCFDia2 or CUL2LRR1, how ubiquitylation of CMG is regulated to restrict replisome disassembly to termination remains a key unanswered question.
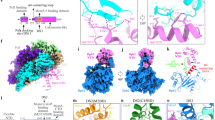
a, Schematic of the regulation of replisome disassembly. For clarity, replisomes are depicted as CMG. CMG ubiquitylation and replisome disassembly are inhibited at replication forks by an as yet unknown mechanism, dependent on the excluded DNA strand (in red box). This inhibition is relieved following translocation onto dsDNA (in green box, left and middle) or off DNA (in green box, right). b, Slice-through view of cryo-EM density for complexes assembled on dsDNA. The density shown is a composite of focused maps (refer to Extended Data Fig. 2). c, DNA engagement within the MCM C-tier motor domains by complexes assembled on dsDNA (coloured) or on a replication fork (grey; PDB: 6SKL13). d, Cryo-EM density as in b (left) and corresponding atomic model (right) for complexes assembled on dsDNA. For the atomic model, only SCFDia2, DNA and MCM subunits that interact with SCFDia2 are coloured. e, Alternative view of the atomic model in d. f, Cryo-EM density for complexes assembled in the absence of DNA, derived from multibody refinement. g, Comparison of the MCM–Dia2LRR interface from complexes assembled on dsDNA (b–e), off DNA (f) or on a replication fork (PDB: 6SKL13). For the regions of MCM at this interface, the root mean square deviation (r.m.s.d.) of the replication fork-bound complex compared with the dsDNA-bound or off-DNA complexes is 1.39 Å and 0.93 Å, respectively.
Terminated yeast replisome structures
To determine the molecular basis for the regulation of CMG ubiquitylation, we aimed to solve the structure of a terminated replisome, by adapting our system for reconstituting budding yeast replisomes for structural analysis13. After convergence of two replication forks, CMG translocates onto nascent double-stranded DNA (dsDNA) produced by the converging replisome3,9,14 (Fig. 1a). To trap a replisome bound around dsDNA, we used a DNA substrate that lacked a 5′ flap and contained a short stretch methylphosphonate modifications embedded in dsDNA, which slow translocation of CMG15 (Extended Data Fig. 1a). This DNA substrate was incubated with CMG, the replisome factors Tof1–Csm3, Mrc1 and Ctf4, SCFDia2 (Hrt1–Cdc53–Skp1–Dia2), an E2–ubiquitinconjugate (Cdc34–Ub)16, and the leading-strand DNA polymerase Pol-ε, in the presence of ATP (Extended Data Fig. 1a). After glycerol gradient sedimentation, complexes containing all replisome and SCFDia2 subunits were isolated (Extended Data Fig. 1b). Cdc34–Ub did not associate with the complex, perhaps reflecting the absence of neddylation on the Cdc53 cullin subunit of SCFDia2 (refs. 17,18).
After gradient fixation, samples were prepared for cryo-electron microscopy (cryo-EM), yielding three-dimensional (3D) reconstructions at average resolutions of 3.2–4.0 Å (Fourier shell correlation (FSC) = 0.143 criterion; Extended Data Fig. 1c–h, Extended Data Table 1). DNA binding was heterogenous across the dataset, with the majority of particles still engaging single-stranded DNA (Extended Data Fig. 2). Nonetheless, we identified a subset of particles, which was subsequently subclassified into two conformations (conformations I and II), that had unambiguously translocated onto dsDNA, representative of bona fide termination intermediates produced after fork convergence (Fig. 1b, Extended Data Figs. 2, 3a–e). While the configuration of the MCM C-tier differed between conformations I and II (Extended Data Fig. 3), in both cases the incoming dsDNA was bent by approximately 46° between the MCM N-tier and C-tier, necessitating distortion of the DNA duplex within the N-tier (Extended Data Fig. 3f). For conformation I, the nucleotide occupancy and interactions with the phosphate backbone of the leading-strand template are similar to replication fork-bound CMG13 (Fig. 1c, Extended Data Fig. 3b, g), suggesting a shared mechanism for translocation of CMG over single-stranded DNA and dsDNA15.
Having identified particles that had translocated onto dsDNA, we were able to build an atomic model of a terminated replisome (Fig. 1d). The overall architecture of CMG, Ctf4, Tof1–Csm3 and the non-catalytic module of Pol-ε (Pol-εnon-Cat) was almost indistinguishable from previous structures13,19,20,21 (for details of the structure of Pol-ε, see Extended Data Fig. 4). We observed an additional, large region of density at the N-tier face of CMG beside Mcm3 and Mcm7, which closely approaches Csm3 and the dsDNA ahead of CMG, before extending away from the core of the complex, forming an elongated arm characteristic of the cullin subunit (Cdc53) of SCFDia2 (Fig. 1d, e). The resolution of the cullin arm is relatively poor (precluding model building for Cdc53–Hrt1), due to a large degree of flexibility in this region, as highlighted by comparison of 3D classes (Extended Data Fig. 5a). We predict that this flexibility is important for conjugating the long K48-linked polyubiquitin chains required for Cdc48-dependent replisome disassembly8. Regardless, the orientation of SCFDia2 can be unambiguously defined, placing the Cdc53 C terminus and Hrt1 ~45–70 Å from the primary ubiquitylation site on Mcm7 (Lys29)8,22 (Extended Data Fig. 5a, b), consistent with previous structures of un-neddylated cullin-RING E3 ligases23.
Density corresponding to the E3 ligase substrate-recognition module (Skp1–Dia2) is adjacent to the N-tier face of CMG (Fig. 1d, e). The N-terminal tetratricopeptide repeat domain of Dia2, which binds Ctf4 and Mrc1 (refs. 8,24,25), was not visible in our structure. However, clearsecondary structure and side chain density enabled us to build a de novo atomic model for the remainder of Dia2, encompassing the F-box (residues 211–247), 15 tandem leucine-rich repeats (LRRs) (248–716) and a C-terminal tail (717–732), which folds back onto the concave surface of the horseshoe-shaped LRRs (Extended Data Fig. 5c–k). The C-terminal end of the LRR domain forms an extensive interface with the N-tier of the Mcm3, Mcm5 and Mcm7 subunits of CMG (Fig. 1d, Extended Data Fig. 5l–n; see text below for a detailed description), demonstrating that Dia2 binds directly to CMG bound around dsDNA, equivalent to the situation after convergence of two replication forks.
When DNA replication terminates at the end of linear chromosomes, CMG is thought to dissociate from DNA, at which point the loss of the excluded strand triggers CMG ubiquitylation8,9 (Fig. 1a). To establish how SCFDia2 engages the replisome following termination at chromosome ends, we repeated cryo-EM sample preparation as described above, except in the absence of DNA. This yielded a 3D reconstruction of an ‘off DNA’ replisome at 3.9 Å resolution (Fig. 1f, Extended Data Fig. 6). Notably, binding of the Dia2 LRRs across Mcm3, Mcm5 and Mcm7 is indistinguishable from complexes bound around dsDNA (Fig. 1g, Extended Data Fig. 3e). Furthermore, comparison of our dsDNA-bound and off-DNA complexes with a previous structure of a replication fork-associated replisome13 revealed no conformational changes in the region of the MCM N-tier to which Dia2 binds (Fig. 1g, Extended Data Fig. 3e). Therefore, we conclude that termination does not induce conformational changes in CMG that are important for the regulation of CMG ubiquitylation by SCFDia2 (ref. 26), either following fork convergence or when CMG dissociates from DNA.
Dia2LRR–MCM interface
The extensive interface between Dia2LRR and MCM is predominantly formed by the Mcm3 N-tier (helices α1 and α5 and the zinc-finger (ZnF) domain), which forms a cradle for the C terminus of Dia2LRR (Fig. 2a, Extended Data Fig. 5l–n). In addition, the N terminus of Mcm7 wraps around the ZnF domain of Mcm3 and becomes sandwiched between Mcm3 and Dia2, while the ZnF domain of Mcm5 interacts with the C-terminal end of Dia2LRR, at the periphery of the Dia2LRR–MCM interface. The details of the residues involved are illustrated in Extended Data Fig. 5n.
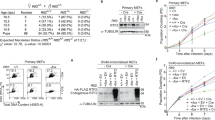
a, Overview of the MCM–Dia2LRR interface. Leading-strand and lagging-strand template DNA is coloured orange and pink, respectively. Residues altered in Dia2LRR mutants are in yellow. b, Reaction scheme to monitor CMG–Mcm7 ubiquitylation after Pif1-stimulated replication fork convergence in vitro14 (left). Immunoblot of reactions conducted as indicated is also shown (right). The experiment was repeated three times. IP, immunoprecipitation; Mut, mutant; Ub, ubiquitin; WT, wild type. c, SDS–PAGE and immunoblotting of TAP–Sld5 immunoprecipitations from G1-arrested yeast cells with the indicated Dia2 alleles. The experiment was repeated twice. Also see Extended Data Fig. 7i. TAP, tandem affinity purification. d, Spot-dilution assay (tenfold serial dilutions) with the indicated yeast strains. The experiment was repeated three times. For gel source data, see Supplementary Fig. 1. YPD, yeast extract peptone dextrose.
To examine the significance of the Dia2LRR–MCM interaction for CMG ubiquitylation and replisome disassembly, we generated a series of point mutants targeting the Dia2LRR–MCM interface, in both Dia2 (Fig. 2a) and MCM. The majority of MCM mutants exhibited defects in the formation of the Mcm2-7–Cdt1 complex or in MCM loading (data not shown), probably because the Dia2 LRR binding site is positioned at the inter-hexamer interface in the MCM double hexamer27. While this precluded analyses of Mcm7 ubiquitylation after convergence of two replication forks in vitro, we were able to purify a CMG complex containing mutations in Mcm3 and Mcm5, which, while being proficient for DNA replication, was defective for ubiquitylation of Mcm7 (Extended Data Fig. 7a–d). Dia2LRR mutants formed stable tetrameric SCFDia2 complexes and supported ubiquitylation of Ctf4 (Extended Data Fig. 7e–g). Importantly, with the exception of Dia2-3A, the Dia2LRR mutants were defective for ubiquitylation of Mcm7, both after replication fork convergence (Fig. 2b) and off DNA (Extended Data Fig. 7h), with Dia2-13A showing the most penetrant defect. Haploid yeast cells with the dia2-13A allele accumulated CMG in the G1 phase of the cell cycle (Fig. 2c, Extended Data Fig. 7i), reflecting a failure to disassemble CMG during replication termination in the S phase of the previous cell cycle1. Furthermore, these cells exhibited a profound growth defect at 20 °C, indistinguishable from cells lacking Dia2 (ref. 24) (Fig. 2d). Together, these data demonstrate that the Dia2LRR–MCM interface that we describe is essential for CMG ubiquitylation and replisome disassembly, both after fork convergence and when CMG dissociates from DNA.
Human replisome–CUL2LRR1 structure
Ubiquitylation of CMG in metazoa is driven by CUL2LRR1 (LRR1–CUL2–ELOB–ELOC–RBX1)4,5,6,7. Although LRR1 displays no apparent sequence homology to Dia2, metazoan CUL2LRR1 ubiquitylates CMG on its MCM7 subunit4,5,6 and is suppressed by the excluded DNA strand9,10, suggesting there might be common features of replisome association that are important for the regulation of both SCFDia2 and CUL2LRR1. To investigate this, we used our approach for human replisome assembly28 and a DNA substrate lacking a 5′ flap, to determine a high-resolution structure of CUL2LRR1 in the human replisome (Fig. 3a, b, Extended Data Figs. 8, 9).
a, Cryo-EM density of the human replisome bound by CUL2LRR1. The density shown is a composite of focused maps (refer to Extended Data Fig. 8). b, Atomic models for the human replisome bound by CUL2LRR1 displayed using transparent surface rendering, except for CUL2LRR1. Only CUL2LRR1, DNA and the CUL2LRR1-interacting regions of MCM are coloured (left). The model indicating the distance between RBX1 and K28/K29MCM7 is coloured according to subunit (right). c, LRR1 domain architecture diagram. The primary sequence and LRRs 1–9 are numbered. PH, pleckstrin homology. d, Overview of the interface between LRR1 and the replisome. The model is displayed using surface rendering, except for LRR1 and DNA.
The overall architecture of human CMG, AND-1, TIMELESS–TIPIN and Pol-ε are indistinguishable from our previous structure lacking CUL2LRR1 (ref. 28) (Fig. 3a, b, Extended Data Fig. 10a). LRR1 is positioned across the MCM N-tier, in close proximity to the parental DNA duplex. In addition, an elongated arm of lower-resolution density, into which thecrystal structure of ELOB–ELOC–CUL2–RBX1 could be unambiguously docked29, projects from the MCM N-tier in an analogous manner to yeast Cdc53–Hrt1 (Fig. 3b). Although metazoan CUL2 and yeast Cdc53 are tethered to their respective substrate adaptors (ELOB–ELOC–LRR1 for CUL2, Skp1–Dia2 for Cdc53) via very different interactions, the cullin C terminus and RING-box protein are similarly located in both cases, ~45–70 Å from the primary ubiquitylation sites in Mcm7 (refs. 8,9,22) (Figs. 1d, 3b). Furthermore, like Cdc53, CUL2 displays considerable conformational variability, which is probably important for the conjugation of long polyubiquitin chains onto MCM7 (refs. 8,30) (Extended Data Figs. 5a, 10b).
The majority of LRR1 was well resolved in our cryo-EM map (Extended Data Fig. 9e, k–n), which enabled de novo modelling of an N-terminal pleckstrin homology domain and a C-terminal LRR domain, which are connected by a flexible linker that stretches perpendicularly across the parental dsDNA (Fig. 3c, d). The pleckstrin homology domain interacts with the ZnF domains of MCM2 and MCM6, parental dsDNA and the N-terminal region of the TIMELESS α-solenoid (Extended Data Fig. 10c, d), consistent with the reported role for TIMELESS–TIPIN in recruiting CUL2LRR1 to the replisome in Caenorhabditis elegans30. The LRR domain comprises seven canonical and two irregular LRR motifs and forms a shallow arc, reaching from the parental dsDNA to the N-tier face of MCM3 and MCM5 (Fig. 3d, Extended Data Fig. 10e, f). The BC and CUL2 boxes, which link LRR1 to ELOB–ELOC–CUL2–RBX1, are situated between LRR repeats 8 and 9 (Fig. 3c, d, Extended Data Fig. 10e, f), and a two-stranded antiparallel β-sheet caps the LRR domain at its C-terminal end (Extended Data Fig. 10g). In addition, the C-terminal HMG box of AND-1 could be docked into a small region of density alongside ELOC and LRR1 (Extended data Fig. 10h, i), which was absent in 3D classes that lacked AND-1 (Extended Data Fig. 10j, k), indicating that AND-1 interacts with CUL2LRR1 in the human replisome.
Remarkably, despite the very different architectures of the LRR1 and Dia2 LRR domains, they bind to the same region of the MCM N-tier, but do so via completely different modes of interaction. The LRR1 LRR domain interacts predominantly with the three-stranded antiparallel β-sheet of the ZnF domain of MCM3, which extends the shallow arc of the LRR1 β-sheet (Extended Data Fig. 10l). This interface is augmented on one side by interactions between the MCM7 N terminus and the tip of the ZnF domain of MCM3 and LRR1 repeats 8 and 9 (Extended Data Fig. 10l, m). On the other side, MCM3 residues 3–8 and 164–174 are significantly rearranged upon CUL2LRR1 binding, such that the N terminus of MCM3, now projecting between the ZnF domains of MCM3 and MCM5, stabilizes an interaction between MCM3 residues 164–174 and a loop and short helix preceding LRR1 repeat 9 (Extended Data Fig. 10n). Finally, charged residues immediately preceding the β-strands of LRR1 repeats 4–7 form multiple polar contacts with the tip of the ZnF domain of MCM5 (Extended Data Fig. 10m). Further details are illustrated in Extended Data Fig. 10m, n.
Regulation of CMG ubiquitylation
Ubiquitylation of CMG by both SCFDia2 and CUL2LRR1 is suppressed by the excluded DNA strand at replication forks8,9,10; our discovery that Dia2 and LRR1 bind directly to a common site across the ZnF domains of MCM3 and MCM5 suggested that this region of MCM might be important for the regulation of ubiquitylation. In our recent structure of the human replisome bound to a replication fork28, cryo-EM density that we attributed to the excluded strand was positioned in the channel between the ZnF domains of MCM3 and MCM5, consistent with previous structures of Drosophila and budding yeast CMG13,31,32. To further validate our assignment of the excluded strand, we identified a subset of particles lacking CUL2LRR1 from our dataset of replisomes assembled without an excluded strand (Extended Data Figs. 8, 9g). In the resulting density map, the MCM N-tier was identical to our previous map of replication fork-associated CMG28, apart from a single region of density, extending from the fork junction between the ZnF domains of MCM3 and MCM5 (Fig. 4a, Extended Data Fig. 10o). This density was present only in the complex associated with the replication fork, thus confirming that it is contributed by the excluded DNA strand.
a, Comparison of cryo-EM density maps for human replisome complexes (CMG, TIMELESS, TIPIN, CLASPIN, AND-1 and Pol-ε) bound to DNA substrates either lacking (left) or featuring (right; EMDB: EMD-13375 (ref. 28)) a 15-nucleotide 5ʹ flap, representing the excluded DNA strand. Density is coloured according to chain occupancy using a radius of 5 Å, with the excluded strand coloured manually in UCSF Chimera. ssDNA, single-stranded DNA. b, Alternative views of the ZnF domains of MCM3 and MCM5 during replication elongation (red box, excluded strand present28) and termination (green box, excluded strand absent). In the upper panel of the red box, the dashed line shows a putative path for the excluded ssDNA beyond the density observed in a, right. In the lower panel of the red box, four sugar-phosphate backbone linkages were built into the excluded strand density (see a, right; EMDB: EMD-13375 (ref. 28)). H. sapiens, Homo sapiens. c, Model for the regulation of CMG ubiquitylation. LRR-interacting regions of MCM are occluded in the MCM double hexamer (see Extended Data Fig. 11a) and by the excluded DNA strand at replication forks (see a, b) (red box). Loss of the excluded strand upon termination allows LRR–MCM engagement, CMG ubiquitylation and replisome disassembly (green box).
Crucially, Fig. 4b shows that the presence of the excluded strand between the ZnF domains of MCM3 and MCM5 sterically blocks the engagement of the Dia2 and LRR1 LRR domains with MCM. As the LRR–MCM interaction is essential for ubiquitylation of CMG and, in turn, replisome disassembly, the occlusion of this interface by the excluded strand provides an elegant and universal explanation for the regulation of replisome disassembly across yeasts and metazoa. Notably, the LRR domains of Dia2 and LRR1 are not demonstrably homologous in sequence or structure. Thus, we propose that the binding of Dia2LRR and LRR1LRR across the exit channel of the excluded strand reflects convergent evolution, probably indicative of a stringent evolutionary pressure to accurately regulate replisome disassembly, and thereby safeguard replication forks. This evolutionary constraint is not evident in parts of the replisome disassembly machinery that do not contribute to the regulation of CMG ubiquitylation. For example, the Dia2 tetratricopeptide repeat domain binds yeast Mrc1 and Ctf4, whereas the LRR1 pleckstrin homology domain binds human TIMELESS.
On the basis of our results, we propose the model summarized in Fig. 4c. Ubiquitylation of the MCM double hexamer is blocked by the occlusion of the LRR binding site at the inter-hexamer interface27 (Extended Data Fig. 11a). This occlusion probably also suppresses ubiquitylation during the conversion of MCM double hexamers into pairs of active CMG helicases33, before the lagging-strand template is excluded. Once bidirectional replication forks are established and elongation begins, the spooling of the excluded DNA strand between the ZnF domains of MCM3 and MCM5 sterically blocks LRR engagement on MCM. It is possible that the binding of proteins to the excluded strand may help to block LRR–MCM engagement. However, ubiquitylation of CMG is inhibited at reconstituted budding yeast replication forks in the absence of the lagging-strand machinery (Extended Data Fig. 11b, c), consistent with the excluded DNA alone being sufficient to suppress SCFDia2 during elongation. In principle, the binding of yeast Dia2 to Mrc1 and Ctf4, and human LRR1 to TIMELESS and AND-1, could still occur at replication forks, even when the LRR–MCM interaction is blocked by the excluded strand. Accordingly, Mrc1–Ctf4 can support SCFDia2-13A recruitment to reconstituted replisomes (Extended Data Fig. 11d, e). Critically, however, the essentiality of the LRR–MCM interaction for ubiquitylation of CMG will restrict replisome disassembly to termination, independent of the timing of E3 ligase recruitment, and irrespective of whether a replication fork terminates via fork convergence, or at a telomere.
Finally, we note that if the excluded strand is ever mispositioned, for example, during replication fork stalling or reversal, replisome disassembly could be triggered, due to premature LRR–MCM engagement. As such, the regulatory mechanism that we describe here may have implications for the stability of the replication fork under conditions of replication stress.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
Cryo-EM density maps of the yeast replisome–SCFDia2 complex on dsDNA have been deposited in the Electron Microscopy Data Bank (EMDB) under the following accession numbers: EMD-13495 (full-complex unsharpened map, conformation I), EMD-13496 (full-complex sharpened map, conformation I), EMD-13497 (multibody refinement (MBR), MCM N-tier, conformation I), EMD-13498 (MBR, MCM C-tier, conformation I), EMD-13500 (full-complex unsharpened map, conformation II), EMD-13512 (full-complex sharpened map, conformation II), EMD-13513 (MBR, MCM N-tier, conformation II), EMD-13514 (MBR, MCM C-tier, conformation II), EMD-13515 (MBR, Dia2–Skp1), EMD-13516 (MBR, Cdc45–GINS–Ctf4–Dpb2NTD), EMD-13517 (MBR, Pol-εnon-Cat–Mcm5WH) and EMD-13518 (full complex enriched for Csm3–Tof1); composite maps produced using Phenix combine_focused_maps have been deposited under accession numbers EMD-13537 (conformation I) and EMD-13539 (conformation II). Cryo-EM density maps of the yeast replisome–SCFDia2 complex in the absence of DNA have been deposited in the EMDB under the following accession numbers: EMD-13519 (full-complex unsharpened map) and EMD-13540 (MBR). Cryo-EM density maps of the human replisome–CUL2LRR1 complex used in model building have been deposited in the EMDB under the following accession numbers: EMD-13494 (full complex, consensus refinement), EMD-13491 (MBR, AND-1–CDC45–GINS), EMD-13490 (MBR, ELONGIN–BC–LRR1–CUL2) and EMD-13492 (MBR, CUL2–RBX1). An additional map of the core human replisome not engaged by CUL2LRR1 on a DNA substrate lacking a 5′ flap has been deposited under the accession number EMD-13534. Atomic coordinates have been deposited in the Protein Data Bank (PDB) with the accession numbers 7PMK for the yeast replisome–SCFDia2 complex on dsDNA (conformation I), 7PMN for the yeast replisome–SCFDia2 complex on dsDNA (conformation II) and 7PLO for the human replisome–CUL2LRR1 complex.
References
Maric, M., Maculins, T., De Piccoli, G. & Labib, K. Cdc48 and a ubiquitin ligase drive disassembly of the CMG helicase at the end of DNA replication. Science 346, 1253596 (2014).
Moreno, S. P., Bailey, R., Campion, N., Herron, S. & Gambus, A. Polyubiquitylation drives replisome disassembly at the termination of DNA replication. Science 346, 477–481 (2014).
Dewar, J. M., Budzowska, M. & Walter, J. C. The mechanism of DNA replication termination in vertebrates. Nature 525, 345–350 (2015).
Dewar, J. M., Low, E., Mann, M., Raschle, M. & Walter, J. C. CRL2Lrr1 promotes unloading of the vertebrate replisome from chromatin during replication termination. Genes Dev. 31, 275–290 (2017).
Sonneville, R. et al. CUL-2LRR-1 and UBXN-3 drive replisome disassembly during DNA replication termination and mitosis. Nat. Cell Biol. 19, 468–479 (2017).
Villa, F. et al. CUL2LRR1, TRAIP and p97 control CMG helicase disassembly in the mammalian cell cycle. EMBO Rep. 22, e52164 (2021).
Fan, Y. et al. LRR1-mediated replisome disassembly promotes DNA replication by recycling replisome components. J. Cell Biol. 220, e202009147 (2021).
Deegan, T. D., Mukherjee, P. P., Fujisawa, R., Polo Rivera, C. & Labib, K. CMG helicase disassembly is controlled by replication fork DNA, replisome components and a ubiquitin threshold. eLife 9, e60371 (2020).
Low, E., Chistol, G., Zaher, M. S., Kochenova, O. V. & Walter, J. C. The DNA replication fork suppresses CMG unloading from chromatin before termination. Genes Dev. 34, 1534–1545 (2020).
Vrtis, K. B. et al. Single-strand DNA breaks cause replisome disassembly. Mol. Cell 81, 1309–1318.e6 (2021).
Labib, K., Tercero, J. A. & Diffley, J. F. Uninterrupted MCM2-7 function required for DNA replication fork progression. Science 288, 1643–1647 (2000).
Fu, Y. V. et al. Selective bypass of a lagging strand roadblock by the eukaryotic replicative DNA helicase. Cell 146, 931–941 (2011).
Baretić, D. et al. Cryo-EM structure of the fork protection complex bound to CMG at a replication fork. Mol. Cell 78, 926–940.e13 (2020).
Deegan, T. D., Baxter, J., Ortiz Bazan, M. A., Yeeles, J. T. P. & Labib, K. P. M. Pif1-family helicases support fork convergence during DNA replication termination in eukaryotes. Mol. Cell 74, 231–244.e9 (2019).
Langston, L. D. & O’Donnell, M. E. An explanation for origin unwinding in eukaryotes. eLife 8, e46515 (2019).
Plechanovova, A., Jaffray, E. G., Tatham, M. H., Naismith, J. H. & Hay, R. T. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature 489, 115–120 (2012).
Kawakami, T. et al. NEDD8 recruits E2-ubiquitin to SCF E3 ligase. EMBO J. 20, 4003–4012 (2001).
Xie, P. et al. The covalent modifier Nedd8 is critical for the activation of Smurf1 ubiquitin ligase in tumorigenesis. Nat. Commun. 5, 3733 (2014).
Goswami, P. et al. Structure of DNA-CMG-Polε elucidates the roles of the non-catalytic polymerase modules in the eukaryotic replisome. Nat. Commun. 9, 5061 (2018).
Zhou, J. C. et al. CMG-Polε dynamics suggests a mechanism for the establishment of leading-strand synthesis in the eukaryotic replisome. Proc. Natl Acad. Sci. USA 114, 4141–4146 (2017).
Sun, J. et al. The architecture of a eukaryotic replisome. Nat. Struct. Mol. Biol. 22, 976–982 (2015).
Maric, M., Mukherjee, P., Tatham, M. H., Hay, R. & Labib, K. Ufd1-Npl4 recruit Cdc48 for disassembly of ubiquitylated CMG helicase at the end of chromosome replication. Cell Rep. 18, 3033–3042 (2017).
Zheng, N. et al. Structure of the Cul1–Rbx1–Skp1–F boxSkp2 SCF ubiquitin ligase complex. Nature 416, 703–709 (2002).
Morohashi, H., Maculins, T. & Labib, K. The amino-terminal TPR domain of Dia2 tethers SCFDia2 to the replisome progression complex. Curr. Biol. 19, 1943–1949 (2009).
Maculins, T., Nkosi, P. J., Nishikawa, H. & Labib, K. Tethering of SCFDia2 to the replisome promotes efficient ubiquitylation and disassembly of the CMG helicase. Curr. Biol. 25, 2254–2259 (2015).
Dewar, J. M. & Walter, J. C. Mechanisms of DNA replication termination. Nat. Rev. Mol. Cell Biol. 18, 507–516 (2017).
Li, N. et al. Structure of the eukaryotic MCM complex at 3.8 A. Nature 524, 186–191 (2015).
Jones, M. L., Baris, Y., Taylor, M. R. G. & Yeeles, J. T. P. Structure of a human replisome shows the organisation and interactions of a DNA replication machine. EMBO J. https://doi.org/10.15252/embj.2021108819 (2021).
Cardote, T. A. F., Gadd, M. S. & Ciulli, A. Crystal structure of the Cul2-Rbx1-EloBC-VHL ubiquitin ligase complex. Structure 25, 901–911.e3 (2017).
Xia, Y., Fujisawa, R., Deegan, T. D., Sonneville, R. & Labib, K. P. M. TIMELESS-TIPIN and UBXN-3 promote replisome disassembly during DNA replication termination in Caenorhabditis elegans. EMBO J. 40, e108053 (2021).
Eickhoff, P. et al. Molecular basis for ATP-hydrolysis-driven DNA translocation by the CMG helicase of the eukaryotic replisome. Cell Rep. 28, 2673–2688.e8 (2019).
Yuan, Z. et al. DNA unwinding mechanism of a eukaryotic replicative CMG helicase. Nat. Commun. 11, 688 (2020).
Champasa, K., Blank, C., Friedman, L. J., Gelles, J. & Bell, S. P. A conserved Mcm4 motif is required for Mcm2-7 double-hexamer formation and origin DNA unwinding. eLife 8, e45538 (2019).
Hogg, M. et al. Structural basis for processive DNA synthesis by yeast DNA polymerase varepsilon. Nat. Struct. Mol. Biol. 21, 49–55 (2014).
Yuan, Z., Georgescu, R., Schauer, G. D., O’Donnell, M. E. & Li, H. Structure of the polymerase ε holoenzyme and atomic model of the leading strand replisome. Nat. Commun. 11, 3156 (2020).
Martin, E. C. et al. LRRpredictor—a new LRR motif detection method for irregular motifs of plant NLR proteins using an ensemble of classifiers. Genes 11, 286 (2020).
Schulman, B. A. et al. Insights into SCF ubiquitin ligases from the structure of the Skp1–Skp2 complex. Nature 408, 381–386 (2000).
Xing, W. et al. SCFFBXL3 ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature 496, 64–68 (2013).
Noguchi, Y. et al. Cryo-EM structure of Mcm2-7 double hexamer on DNA suggests a lagging-strand DNA extrusion model. Proc. Natl Acad. Sci. USA 114, E9529–E9538 (2017).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Acknowledgements
We are grateful to R. T. Hay and J. E. Sale for critical feedback on the manuscript; V. Chandrasekharan, T. Dendooven, C. K. Lau and M. E. Wilkinson for practical advice on data processing and model building; S. Chen, G. Sharov, A. Yeates and B. Ahsan for smooth running of the MRC LMB EM facility; J. Grimmett and T. Darling for maintenance of scientific computing facilities; J. Shi for operation of the baculovirus facility; L. Sanchez-Pulido for discussion relating to the evolutionary conservation of Dia2 and LRR1; and C. Polo Rivera and A. Wood for help with flow cytometry analysis. We acknowledge Diamond for access to and support of the cryo-EM facilities at the UK National Electron Bio-Imaging Centre (eBIC), proposals BI23268-20 and BI24557-4, funded by the Wellcome Trust, Medical Research Council (MRC), and Biotechnology and Biological Sciences Research Council (BBSRC). We are grateful to Y. Chaban, O. Raschdorf, S. Masiulis and D. Clare for providing support in the use of the facilities at the eBIC. Ubiquitin and yeast Uba1 were provided by A. Knebel (MRC PPU, Dundee) and antibodies were provided by MRC PPU Reagents and Services (https://mrcppureagents.dundee.ac.uk) unless otherwise stated. This work was supported by the MRC, as part of UK Research and Innovation (MRC grants MC_UP_1201/12 to J.T.P.Y. and MC_UU_12016/13 to K.P.M.L.) and the Wellcome Trust (reference 204678/Z/16/Z for a Sir Henry Wellcome Postdoctoral Fellowship to T.D.D.).
Author information
Authors and Affiliations
Contributions
M.J.-B. performed yeast cryo-EM sample preparation, data acquisition and processing, model building, purification of yeast replisome proteins, created figures, and reviewed and edited the manuscript. M.L.J. performed human cryo-EM sample preparation, data acquisition and processing, model building, created figures, and reviewed and edited the manuscript. Y.B. performed purification of human replisome proteins. K.P.M.L. took part in discussion, acquired funding, and reviewed and edited the manuscript. G.C. acquired the human cryo-EM data. J.T.P.Y. conceptualized and supervised the study, acquired funding, performed yeast biochemistry and optimization of human replisome assembly, purification of yeast and human replisome proteins, wrote the original draft, and reviewed and edited the manuscript. T.D.D. conceptualized and supervised the study, acquired funding, performed yeast biochemistry and genetics, yeast cryo-EM sample preparation, purification of yeast replisome proteins, wrote the original draft, and reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Andrew Deans and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Supporting data for cryo-EM investigation of S. cerevisiae dsDNA-bound replisome:SCFDia2 complexes.
a, Schematic of reconstitution approach used for preparation of cryo-EM sample representing terminating SCFDia2-bound replisome complexes after translocation onto dsDNA. A schematic of the DNA substrate used is shown in orange, with the 20 nt tract of methylphosphonate (MEP) linkages coloured red. b, Silver-stained SDS-PAGE gels analysing 100 μL fractions taken across 10-30% glycerol gradients, either lacking (top) or containing (bottom) crosslinking agents. Fractions 13+14 used for cryo-EM sample preparation are indicated. * = Cdc34-Ub; ** = Cdc34. Similar results were observed for three independent sample preparations. c, Representative cryo-EM micrograph. d, Representative 2D class averages, 40 nm box width. e, Representative angular distribution of particle orientations. A correspondingly oriented model is shown to the right for reference. f, Fourier shell correlation graphs for maps used in model building. The resolution of reconstructions calculated at the FSC=0.143 criterion are reported in Extended Data Fig. 2. g, Model-to-map correlation graphs. h, Cryo-EM density maps relevant to model building, coloured by local resolution. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 2 Data processing pipeline related to S. cerevisiae replisome:SCFDia2 complexes.
The approach used to subclassify complexes based on MCM C-tier conformation and DNA engagement are coloured orange; the approach used to derive cryo-EM reconstructions for model building or adjustment of regions outside MCM are coloured magenta. Reported resolutions are calculated based on the FSC=0.143 criterion (refer to Extended Data Fig. 1f).
Extended Data Fig. 3 DNA engagement by S. cerevisiae CMG following translocation onto dsDNA.
a, Comparison of conformations I and II demonstrating the similarity in the overall complex architecture. For clarity, conformation I is rendered as a surface, whilst conformation II is shown as a cartoon. b, Overview of MCM C-tier domains bound to dsDNA, highlighting ATPase site occupancy in conformations I and II. c, Cryo-EM density (grey) at the MCM C-tier Mcm3-Mcm5 interface for each conformation. Mcm3, cyan; Mcm5, blue; AMP-PNP, red. d, Changes in ATPase site occupancy between conformations I and II correspond to movement of Mcm3/5/7 AAA+ domains and their DNA-binding loops (helix-2-insertion, H2I; presensor-1, PS1). Arrows indicate the relative movement of Mcm5 and Mcm3. The outward movement of Mcm3/Mcm7 in conformation II - associated with opening of the Mcm3/5 interface and loss of nucleotide at this ATPase site - leads to loss of the canonical contacts (described in panel g) formed between the Mcm3 H2I/PS1 loops and the leading-strand template DNA phosphate backbone. As such conformation II may reflect a partially disengaged state. e, Comparison of MCM:Dia2LRR interface between conformations I and II, demonstrating lack of conformational changes in this region. f, Model of DNA in cryo-EM density (mesh) for conformation I demonstrating distortion of the B-form DNA duplex within the MCM N-tier; similar DNA density is observed within the MCM N-tier for conformation II. Approximate trajectories of DNA strands within the MCM N-tier are shown as dotted paths. g, Engagement of the leading-strand template DNA phosphate backbone by Mcm H2I/PS1 loops in complexes that have translocated onto dsDNA is comparable to that previously observed for CMG bound to ssDNA13. Contacts shown for the representative Mcm6 subunit (conformation I). Specific contacts with the DNA phosphate moieties indicated by dashed yellow lines.
Extended Data Fig. 4 Insights into Pol ε positioning within the S. cerevisiae replisome.
a, Cryo-EM density derived from multi-body refinement (top) and corresponding atomic model (bottom) of the Pol ε non-catalytic module (Pol εnon-Cat), with the exception of the Dpb2 NTD. b, Representative cryo-EM density (mesh) allowing de novo model building and adjustment of prior structures. c, Pol2-Mcm2 AAA+ domain interface. d, Interactions formed by the Mcm5 winged-helix (WH) domain with Pol εnon-Cat. The Mcm5 WH is observed to contact regions of Pol2 (dark green) in addition to the Pol2 CysB and Dpb2 OB-fold domains. Interactions depicted in c and d have not been characterised previously. e, Regions of additional cryo-EM density observed for SCFDia2-bound replisome complexes on dsDNA, visible at low map contour levels. The crystal structure of the Pol2 catalytic domain (PDB: 4M8O34) has been rigid-body fitted to additional density beside the MCM channel exit. In contrast to previous structures19,28,35, this positions the Pol2 catalytic domain at the C-tier face of CMG, adjacent to the leading-strand template, in a state that may be important for leading-strand synthesis. Additional unassigned density between Ctf4SepB, Tof1 and SCFDia2 is outlined. f, Focused view of additional density attributed to the Pol2 catalytic domain (as in e, except rotated 180°). The C-terminal residue of the Pol2 catalytic domain (residue 1186), the N-terminal residue of the Pol2 non-catalytic domain (residue 1321), and density linking the two domains are indicated.
Extended Data Fig. 5 Supporting information for the Dia2 structure and its interaction with the S. cerevisiae replisome.
a, Cryo-EM density for Cdc53-Hrt1 for two 3D classes following signal subtraction/3D subclassification, demonstrating the flexibility observed in the position of replisome-bound SCFDia2. The position of Hrt1 is shown, derived from rigid-body fitting the crystal structure of homologous CUL1-RBX1 (PDB: 1LDK23). The approximate distance between Hrt1 and the primary ubiquitylation site (K29Mcm7) is indicated. b, Cryo-EM density map for Cdc53-Hrt1 with the crystal structure of homologous CUL1 (PDB: 1LDK23) rigid-body fitted. c, Representative cryo-EM density (mesh) across different regions of Dia2. d, Dia2 domain architecture; TPR domain and nuclear localisation signal (NLS) as in ref. 24. e, f, Alternative views of the Dia2 LRR domain coloured by repeat. The F-box domain and C-terminal tail are shown for context in e. g, Comparison of Dia2 LRR domain repeats to the LRR consensus sequence36. L is Leu/Val/Ile/Phe, N is Asn/Thr/Cys, x is any amino acid; we consider L0 as the first repeat residue. The core LxxLxL motif is highlighted. h, Comparison of Dia2 F-box to the consensus sequence37. Exact matches coloured red, conservative differences coloured green. i, The Dia2 LRR domain (repeats 1 and 2) closely approaches the parental dsDNA (orange: leading-strand template; pink: lagging-strand template) and Csm3. The region of Csm3 upstream of the DNA-binding motif (DBM) is observed to interact with the Dia2 LRR domain β-sheet and Dia2 C-terminal tail, however cryo-EM density for this region was insufficient to identify details of this interaction. Dia2 residues which are positioned close to DNA are labelled. j, Dia2-Skp1 interaction. k, Interactions of Skp1 with alternative LRR-domain-containing F-box proteins, to show similarity with j. Human SKP1-SKP2: PDB:1FQV37; human SKP1-FBXL3: PDB: 4I6J38. l, Overview of interaction between Dia2 LRR domain and Mcm subunits. m, The MCM:Dia2LRR interaction does not involve the concave β-sheet surface of Dia2LRR. n, Detail of the MCM:Dia2LRR interface. Residues altered in Dia2LRR mutants are yellow. For the Mcm3 ZnF, interaction networks are subdivided into those above (A) and below (B) the position of the Mcm7 N-terminus.
Extended Data Fig. 6 Supporting data for cryo-EM investigation of S. cerevisiae replisome:SCFDia2 complexes assembled in the absence of DNA.
a, Silver-stained SDS-PAGE gel analysing 100 μL fractions taken across a 10-30% GraFix gradient. Fractions 1-17 (of 23) shown. Fractions 11-13 used for cryo-EM sample preparation are indicated. Large-scale sample preparation was performed once; similar results were observed in three independent small-scale gradient preparations. b, Representative cryo-EM micrograph. c, Representative 2D class averages, 40 nm box width. d, Angular distribution of particle orientations contributing to cryo-EM density map (Fig. 1f). e, Fourier shell correlation curve for the multi-body refinement map presented in Fig. 1f. f, Cryo-EM density map (related to Fig. 1f) coloured by local resolution. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 7 Supporting data for functional analyses of Dia2 and MCM mutants.
a, Coomassie-stained SDS-PAGE gel of purified CMG complex containing mutations in Mcm3 and Mcm5 at Dia2LRR-MCM interface b, Reaction scheme for in vitro replication of 9.7 kb forked DNA template using CMG and the indicated replication proteins. c, Reaction conducted as in panel b with wildtype or mutant CMG. Samples were separated on an alkaline agarose gel and visualised by auto-radiography. This experiment was performed twice. d, In vitro CMG ubiquitylation reaction in the absence of DNA. The indicated proteins were incubated in the presence of ubiquitin and ATP and then visualised by SDS-PAGE and immunoblotting. This experiment was repeated three times. e, Positions of residues mutated in Dia2 LRR domain. LRR repeats 12-15 are coloured and numbered as in Extended Data Fig. 5e, f. Residues are coloured according to the Dia2 mutant in which they are present; all residues shown were mutated in Dia2-13A. Dia2-8A featured the following mutations: D632A, F657A, I662A, Y665A, Q694A, I698A, T699A and Y716A. f, Coomassie-stained SDS-PAGE gel of purified SCFDia2 complexes containing Dia2LRR mutants g, In vitro Ctf4 ubiquitylation reaction. The indicated proteins were incubated in the presence of ubiquitin and ATP and then visualised by SDS-PAGE and immunoblotting. The Hrt1 immunoblot serves as a loading control for SCFDia2. This experiment was repeated twice. h, Reaction conducted as in panel d with the indicated Dia2LRR mutants. This experiment was repeated three times. i, DNA content of G1-arrested cells from experiment in Fig. 2c was monitored by flow cytometry after propidium iodide staining. The proportion of G1 cells, expressed as a percentage of the total cells, is given. For details of gating strategy and assignment of the G1 peak see Supplementary Fig. 2. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 8 Data processing pipeline related to H. sapiens replisome:CUL2LRR1 complexes.
Each pathway describing the generation of a discrete reconstruction is given its own colour. The final reconstructions are coloured red and boxed.
Extended Data Fig. 9 Supporting data for cryo-EM investigation of H. sapiens replisome: CUL2LRR1 complexes.
a, Schematic of reconstitution approach used for preparation of replisomes bound to CUL2LRR1 for cryo-EM. A schematic of the DNA substrate used is shown with a 39 nucleotide 3′ arm and no 5′ arm. b, Silver-stained SDS-PAGE gels analysing 100 μL fractions taken across 10-30% glycerol gradients, either lacking (top) or containing (bottom) crosslinking agents. Fractions 15+16 used for cryo-EM sample preparation are indicated. This experiment was performed twice. c, Representative cryo-EM micrograph. d, Representative 2D class averages, 40 nm box width. e–j, (Top) cryo-EM reconstructions coloured by local resolution according to inset keys (Bottom) angular distribution of particle orientations. e, Consensus refinement for replisome:CUL2LRR1 fully engaged. f, Consensus refinement for replisome:CUL2LRR1 where the LRR1PH domain is bound but the LRRs are disengaged. g, Consensus refinement for particles lacking CUL2LRR1. h, Multi-body refinement for AND-1:CDC45:GINS. i, Multi-body refinement for LRR1:ELOB:ELOC:CUL2:AND-1-HMG. j, Multi-body refinement for CUL2:RBX1. k, Cryo-EM density for the LRR1 LRRs. l, Cryo-EM density for the LRR1 PH domain. m, Representative cryo-EM density for a LRR1 LRR domain β-strand at 3.5 Å resolution. n, Representative cryo-EM density for a LRR1 LRR domain α-helix at 3.7 Å resolution. o, Fourier-shell correlation (FSC) curves for the various maps used in model building. p, Map-to-model FSC curves for the complete model docked into the consensus refinement for replisomes fully engaged by CUL2LRR1. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 10 Supporting information for the CUL2LRR1 structure and its interaction with the H. sapiens replisome.
a, Structural overlay of aligned model from replisomes bound to CUL2LRR1 (blue) and in the absence of CUL2LRR1 (red). b, Composite model and map representing the conformational variability of CUL2/RBX1. The model for the replisome, bound to LRR1 and ELOB-ELOC, is displayed using pipes and planks rendering and coloured according to subunit. Three representative 3D classes are displayed encompassing density for CUL2:RBX1 obtained through 3D classification without alignment. The distance between RBX1 and K29MCM7 is indicated as a dotted orange line and distances denoted in the inset key. c, Overview of the interface between the LRR1 PH domain and the replisome. Subunits interacting with the LRR1 PH domain are displayed using transparent surface rendering. Boxed regions indicate key interaction interfaces expanded in panel d. d, Detailed structural views of the interface between the LRR1 PH domain and 1: TIMELESS, 2(A): MCM6 ZnF, 2(B): dsDNA and 3: MCM2 ZnF. e, Model for the LRR1 LRRs with numbering indicating the order of the leucine-rich repeats. f, Consensus motif for the LRR1 LRRs. The sequence of each repeat is indicated with the positions of the key L0, L3 and L5 residues highlighted in red. Repeats 1 and 9 represent irregular LRRs. L is Leu/Val/Ile/Phe, N is Asn/Thr/Cys, x is any amino acidL is Leu/Val/Ile/Phe, N is Asn/Thr/Cys, x is any amino acid. g, LRR1 model docked into transparent cryo-EM density with the capping 2-stranded β-sheet highlighted in gold. h, Overview of the LRR1:ELOB:ELOC:CUL2:AND-1 interface. Models displayed docked into transparent cryo-EM density with MCM subunits visualised using surface rendering. i, Structure of the AND-1 HMG box (PDB:2D7L) docked into the AND-1-dependent cryo-EM density adjacent to ELOC and LRR1. Selected hydrophobic core residues displayed. j, Map of the replisome bound to CUL2LRR1 in the absence of AND-1 coloured according to subunit. k, Cryo-EM density of the LRR1:ELOB:ELOC:CUL2 interface obtained through multi-body refinement from particles lacking AND-1. The density attributed to the AND-1 HMG box is dependent upon AND-1. l, Overview of the MCM:LRR1LRR interface. MCM subunits displayed with additional transparent surface rendering and the order of the LRR1LRRs numbered. Red-dashed boxes indicate key interaction sites, expanded in panel m. m, Detail of the MCM:LRR1LRR interface involving contacts between the LRR1LRRs and 1 - MCM3, 2 - MCM5 ZnF and 3 - the MCM7 N-terminus. n, Model highlighting local rearrangements of MCM3 upon binding CUL2LRR1. Structures in the absence (top) and presence (bottom) of CUL2LRR1, coloured according to inset key, highlight the rearrangement of MCM3(1-9) and MCM3(164-174). o, Comparison of the CUL2LRR1-interacting regions of MCM from complexes assembled on a DNA substrate either lacking a 5′-flap (green) or containing a 15 nucleotide 5′-flap (gold, PDB: 7PFO28). Complexes lacked CUL2LRR1. The r.m.s.d. between the two structures for the region shown is 0.43 Å.
Extended Data Fig. 11 Supporting data for model for regulation of CMG ubiquitylation.
a, The MCM:Dia2LRRinterface is occluded in the inactive Mcm2-7 double hexamer. The structure of the budding yeast Mcm2-7 double hexamer is shown (PDB: 5BK4 (ref. 39)): one Mcm2-7 hexamer is displayed as a cartoon, the other as a surface. Double-stranded DNA is coloured orange. The positions of the N-tier (N) and C-tier (C) are labelled for each hexamer. Inset: focused view of the regions of Mcm2-7 involved in interaction with the Dia2 LRR domain, demonstrating the inaccessibility of these regions to Dia2 in the context of a double hexamer. b, Reaction scheme for experiment in panel c, to monitor the suppression of CMG ubiquitylation by DNA in the absence of the indicated proteins (top), which are predicted to interact with the excluded DNA strand during lagging strand synthesis. Pif1 was omitted to block fork convergence. DNase was included after the replication step to release the replisome from DNA, which triggers CMG ubiquitylation8. c, Reaction conducted as in panel b and analysed by SDS-PAGE and immunoblot. This experiment was repeated twice. d, Reaction scheme for experiment in panel e, to monitor the interaction of SCFDia2 with the replisome. e, Reaction conducted as in panel d and analysed by SDS-PAGE and immunoblot. * is rabbit IgG. This experiment was repeated twice. For gel source data, see Supplementary Fig. 1.
Supplementary information
Supplementary Information
This file contains Supplementary Methods; Supplementary Tables 1 and 2; Supplementary Figures 1 and 2; and Supplementary References.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jenkyn-Bedford, M., Jones, M.L., Baris, Y. et al. A conserved mechanism for regulating replisome disassembly in eukaryotes. Nature 600, 743–747 (2021). https://doi.org/10.1038/s41586-021-04145-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04145-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.