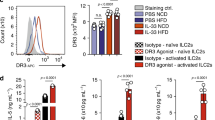
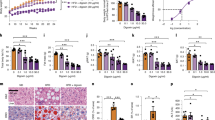
Abstract
Thermogenesis in brown and beige adipose tissue has important roles in maintaining body temperature and countering the development of metabolic disorders such as obesity and type 2 diabetes1,2. Although much is known about commitment and activation of brown and beige adipose tissue, its multiple and abundant immunological factors have not been well characterized3,4,5,6. Here we define a critical role of IL-27–IL-27Rα signalling in improving thermogenesis, protecting against diet-induced obesity and ameliorating insulin resistance. Mechanistic studies demonstrate that IL-27 directly targets adipocytes, activating p38 MAPK–PGC-1α signalling and stimulating the production of UCP1. Notably, therapeutic administration of IL-27 ameliorated metabolic morbidities in well-established mouse models of obesity. Consistently, individuals with obesity show significantly decreased levels of serum IL-27, which can be restored after bariatric surgery. Collectively, these findings show that IL-27 has an important role in orchestrating metabolic programs, and is a highly promising target for anti-obesity immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Our RNA-seq data are available at the Sequence Read Archive (SRA) repository at NCBI under the accession numbers SRX10969398–SRX10969403. Source data are provided with this paper.
References
Betz, M. J. & Enerback, S. Human brown adipose tissue: what we have learned so far. Diabetes 64, 2352–2360 (2015).
Bartelt, A. & Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10, 24–36 (2014).
Wang, W. & Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691–702 (2016).
Odegaard, J. I. et al. Perinatal licensing of thermogenesis by IL-33 and ST2. Cell 166, 841–854 (2016).
Qiu, Y. et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308 (2014).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643 (2017).
Lackey, D. E. & Olefsky, J. M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 12, 15–28 (2016).
Hoffmann, T. J. et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics 210, 499–515 (2018).
Vargas-Alarcon, G. et al. Interleukin 27 polymorphisms, their association with insulin resistance and their contribution to subclinical atherosclerosis. The GEA Mexican study. Cytokine 114, 32–37 (2019).
Yoshida, H. & Hunter, C. A. The immunobiology of interleukin-27. Ann. Rev. Immunol. 33, 417–443 (2015).
Yang, B. et al. IL-27 facilitates skin wound healing through induction of epidermal proliferation and host defense. J. Invest. Dermatol. 137, 1166–1175 (2017).
Nam, H., Ferguson, B. S., Stephens, J. M. & Morrison, R. F. Modulation of IL-27 in adipocytes during inflammatory stress. Obesity 24, 157–166 (2016).
Wolfrum, C. & Straub, L. G. Lessons from Cre-mice and indicator mice. Handb. Exp. Pharmacol. 251, 37–54 (2019).
Kissig, M., Shapira, S. N. & Seale, P. SnapShot: brown and beige adipose thermogenesis. Cell 166, 258–258 (2016).
Kajimura, S. & Saito, M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Ann. Rev. Physiol. 76, 225–249 (2014).
Owaki, T., Asakawa, M., Fukai, F., Mizuguchi, J. & Yoshimoto, T. IL-27 induces Th1 differentiation via p38 MAPK/T-bet- and intercellular adhesion molecule-1/LFA-1/ERK1/2-dependent pathways. J. Immunol. 177, 7579–7587 (2006).
Cao, W. et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 24, 3057–3067 (2004).
Cao, W., Medvedev, A. V., Daniel, K. W. & Collins, S. β-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J. Biol. Chem. 276, 27077–27082 (2001).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Imai, T. et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91, 521–530 (1997).
Rosenwald, M., Perdikari, A., Rulicke, T. & Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 (2013).
Zhang, S. et al. High susceptibility to liver injury in IL-27 p28 conditional knockout mice involves intrinsic interferon-gamma dysregulation of CD4+ T cells. Hepatology 57, 1620–1631 (2013).
Acknowledgements
We thank A. Xu from Hongkong University, L. Ye from the Third Military Medical University, B. Li from Shanghai Jiao Tong University, Y. Qiu from Peking University, Y. Huo from Guangzhou Medical University and J. Chen from Sun Yat-Sen University for discussion, comments and technical guidance; and C. Cote for English language editing of this manuscript. This work is supported by the National Key Research and Development Program of China (no. 2020YFA0803502 to Z.Y.; no. 2017YFA0205200 to L.L.), the National Natural Science Foundation of China (nos 31830021, 32030036 and 31420103901 to Z.Y.; no. 81771957 to L.L.; no. 32070121 to H.Y.; no. 31800721 to Q. Wang; and no. 31500742 to Q.S.), the 111 Project (no. B16021 to Z.Y.), the Incubating Program from the Science and Technology Department of Guangdong Province of China (no. 2014A030308003 to Z.Y.), China Postdoctoral Science Foundation (nos 2018M633278 and 2020M683159 to Q. Wang; no. 2020M673045 to H.Z.).
Author information
Authors and Affiliations
Contributions
Q. Wang, D.L., G.C. and Q.S. conceived this research. Q. Wang, D.L., J.Z., M.Z., H.C. and Q. Wen performed experiments and analysed data. Q.S., C.W. and C.Y. analysed the human samples. H.X. helped with HFD treatment. L. Zhu, H.Z., B.W. and G.L. facilitated in western blot experiments. R.J.P. carried out the metabolic cage experiments and O.S. assisted with cold challenge experiments. Y.Y., S.H. and Y.C. provided technical support for primary adipocyte culture. Z.L. helped in ordering reagents. X.W., Q.Z., Z.J., H.L. and Y.X. assisted in the revision of the manuscript. L. Zhou provided the cold room. C.W. carried out the gastric bypass surgery. X.Y., Y.L., G.I.S. and V.D.D. helped in conceiving the project and afforded guidance in discussions. Q. Wang, G.C. and Z.Y. wrote the manuscript with help from D.L., Q.S., J.Z. and M.Z. L.L., H.Y., R.A.F. and Z.Y. helped in conceiving the project, mentored and supervised its participants.
Corresponding authors
Ethics declarations
Competing interests
Z.Y., Q. Wang and H.Y. have applied a patent with China National Intellectual Property Administration, with Jinan University as the applicant, Z.Y., Q. Wang and H.Y. as the inventors. The application number is 202110986914.X, and the patent covers the screening of IL-27Rα agonists for promoting UCP1 production. The other authors (D.L., G.C., Q.S., J.Z., M.Z., H.C., Q. Wen, H.X., L. Zhu, H.Z., R.J.P., O.S., Y.Y., S.H., Y.C., B.W., G.L., Z.L., C.Y., X.W., L. Zhou, Q.Z., Z.J., H.L., Y.X., X.Y., C.W., Y.L., G.I.S., V.D.D., L.L. and R.A.F.) declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewers for their contribution to the peer review of this paper.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Reduced serum IL-27 level in obese subjects.
a. Serum from obese human subjects (n = 42) and lean healthy controls (n = 26) were used for detection of inflammatory factors by Bio-Rad Bio-Plex 200 Multiplexing Analyzer System. The body mass index (BMI) of individual subject was presented in the first panel. b. Serum IL-27 level in obese patients was detected by ELISA as in Figure 1a. Correlation of BMI and serum IL-27 level in obese subjects was analysed and shown (n = 32). c. Correlation of fasting glucose and serum IL-27 level in type 2 diabetes (T2D) patients (n = 12). Data are mean ± s.d. of biologically independent samples. Two-tailed unpaired student’s t-test (a); linear regression analysis (b & c). NS, not significant.
Extended Data Fig. 2 IL-27Rα deficiency aggravates HFD-induced obesity.
IL-27Rα KO and WT mice at 8 weeks of age were fed on HFD or ND for 10 weeks (a–f). a. Histology (H&E) slides of epididymal white adipose tissue (eWAT), subcutaneous white adipose tissue (SCW) and brown adipose tissue (BAT). Scale bar = 50µm. b. Histology (H&E) and oil red O staining of liver from HFD treated mice. Scale bar = 100µm. Serum was collected after 10 weeks HFD treatment and triglyceride (c, n=6), (d) leptin (n = 5) and adiponectin (n = 4 for WT and n = 6 for KO) were detected. e. The infiltrated macrophages in the stromal vascular fraction (SVF) of epididymal fat were analysed by FACS, the statistical analysis of macrophage percentage in CD45+ is shown (n = 8 for WT and n = 11 for KO). f. Realtime PCR analysis of key cytokines in epididymal fat (eWAT) from mice fed on HFD for 10 weeks (n = 12 for WT-Il4 and n = 10 for KO-Il4, n = 13-WT and n = 12-KO for the rest genes). g. C57BL/6J, IL-27Rα KO and C57BL/6N mice were genotyped via Nnt gene PCR. h–j. IL-27Rα KO and C57BL/6N mice at 8 weeks of age were fed on HFD for 10 weeks. h. Body weight was recorded each week (n = 14 for C57BL/6N and n = 10 for KO). i. Adipose tissues and livers were collected and weighted (n=5). j. GTT was performed (n = 14 for C57BL/6N and n = 10 for KO). k. The splenocytes were isolated from Il27ra+/+, Il27ra+/- and Il27ra-/- mice and protein samples were used for immunoblotting analysis of IL-27Rα. Each lane represents one biological independent sample. l–n. Il27ra+/- and Il27ra+/+ mice at 8 weeks of age were fed on HFD for 10 weeks. l. Body weight was recorded each week (n = 10 for Il27ra+/+ and n = 5 for Il27ra+/-). GTT (m, n = 3 for Il27ra+/+ and n = 6 for Il27ra+/-) and ITT (n, n = 4 for Il27ra+/+ and n = 7 for Il27ra+/-) were performed. o. IL-27Rα KO (n = 18) and WT (n = 12) mice at 8 weeks of age were fed on HFD, GTT was performed after 4 weeks of HFD treatment. All experiments were repeated at least twice with similar results. Data are mean ± s.e.m. of biologically independent samples. Two-tailed unpaired student’s test (c–f & i); two-way ANOVA (h); two-way ANOVA with Sidak’s multiple comparisons test (j & l–o). NS, not significant.
Extended Data Fig. 3 Reduced energy expenditure and thermogenesis in IL-27 signalling deficient mice.
a–c. IL-27Rα KO and WT mice at 8 weeks of age were placed in metabolic cages. The food intake (a, n = 8 for WT and n = 7 for KO), oxgen consumption (b, n = 8) and energy expenditure (c, n = 8) were shown. d. BAT and SCW tissues were isolated from WT-ND and IL-27Rα KO-ND mice at 6-8 weeks of age, cut into small pieces (~0.003g for BAT and ~0.004g for SCW) and used for detection of basal oxygen consumption rate by Seahorse XF Analyzer (n = 5 mice per group, 18 pieces for WT-BAT, 23 pieces for KO-BAT and 17 pieces for SCW samples). e & f. IL-27Rα KO and WT mice at 8 weeks of age were fed on HFD for 10 weeks, SCW were collected for RNA-seq analysis. e. Gene set enrichment analysis was performed for indicated pathways. Genes were ranked according to their expression. NES, normalized enrichment score; FDR, false discovery rate. f. Heat maps of differentially expressed genes in indicated pathway. g. Gene expression in SCW from IL-27Rα KO and WT mice on normal diet were determined by real-time PCR (n = 9 for WT-Ucp1, WT-Cidea, WT-Adipoq and WT-Retn, n = 10 for WT-Ppargc1a, WT-Ppara, WT-Cox8b, WT-Cox5a, WT-Prdm16 and WT-Elovl3, n = 11 for WT-Pparg, WT-Fabp4 and WT-Lep, n = 8 for KO-Ucp1, n = 10 for KO-Retn, n=11 for the rest KO mRNAs). h. Survival curve of IL-27Rα KO and WT mice fed on ND in response to cold challenge (4 °C, n = 9 for WT and n=11 for KO). i. Rectal temperature of mice fed on ND in response to cold challenge (4 °C, n = 9 for WT and n = 11 for KO). j–l. EBI-3 KO mice and WT controls fed on normal chow were challenged at 4 °C. The survival curve (j) and rectal temperature (k) were recorded and shown (n = 5 for WT and n=6 for KO). After 12 hours of cold stimulation, BAT and SCW were collected, lysed and used for immunoblotting analysis of UCP1 (l). m-p. EBI-3 KO mice were i.p. injected with rmIL-27 (100µg/kg) or PBS every day for 7 days and then challenged at 4°C. m. Rectal temperature of mice in response to cold was shown (n = 6). n. Immunoblot analysis of protein extracts from SCW and BAT after 24 hours of cold challenge. UCP1 staining (o) and histology analysis (p) (Scale bar = 100µm). Representative sections were shown. (l & n) Each lane represents one biological independent sample and band densities were quantified with ImageJ, ratios of UCP1/HSP90 were normalized. All experiments were repeated at least twice with similar results. Data are mean ± s.e.m. Data represent biologically independent samples except for d. Two-tailed unpaired student’s t-test (d & g); two-way ANOVA (a–c); two-way ANOVA with Sidak’s multiple comparisons test (i, k & m); log-rank test (h & j); Gene Set Enrichment Analysis (e).
Extended Data Fig. 4 The counteracting obesity effects of IL-27Ra signalling was not through direct actions on CD2+ lymphoid or Lyz2+ myeloid cells.
a. Schematic model for the generation of Il27raflox/flox mice. The Il27ra locus (top) was targeted by the targeting vector (second), which contains the homologous sequence of Il27ra, including two LoxP sites flanking exons 3&4, and a Neo selecting cassette. The linearized vector was subsequently delivered to embryonic stem cells (C57BL/6) via electroporation, followed by drug selection, PCR screening, and Southern Blot confirmation. Homologous recombination resulted in the floxed allele (Third). After confirming correctly targeted ES clones via Southern Blotting, selected clones were used for blastocyst microinjection to produce the F0 generation. The F1 were confirmed as germline-transmitted via crossbreeding F0 with Flp-deleter to delete Neo cassette (Fourth). After Cre recombination, the floxed Il27ra allele will results in the deletion of exons 3 and 4 (bottom). b. Genotyping of Il27raf/f mice. c. IL-27Rα expression in spleen from Il27raf/f and WT mice were determined by Real-time PCR (n = 6, left) and Western Blot (right, each lane represents one biological independent sample). d. Il27ra gene expression in FACS sorted CD3+ T cells, CD45+ CD19+ B cells, CD45+ Lin- (CD3- CD4- CD8a- CD19- CD11b- Ly6c-) CD90.2+ CD127+ ILCs, CD45+ CD3- NK1.1+ NK cells from the spleen of Il27raf/f and Cd2-Cre Il27raf/f mice were determined by Real-time PCR (n = 6, left 4 panels). Thioglycollate was intraperitoneally injected into Il27raf/f and Lyz2-Cre Il27raf/f mice for 4 days, and then the peritoneal macrophages were collected for the detection of Il27ra gene expression by Real-time PCR (n = 6, last panel). e-h. Il27raf/f, Cd2-Cre Il27raf/f and Lyz2-Cre Il27raf/f mice at 8 weeks of age were fed on HFD for 10 weeks. e. Body weight was recorded each week (n = 21 for Il27raf/f, n=11 for Cd2-Cre Il27raf/f and n = 18 for Lyz2-Cre Il27raf/f). GTT (f, n = 15 for Il27raf/f, n = 11 for Cd2-Cre Il27raf/f and n = 19 for Lyz2-Cre Il27raf/f) and ITT (g, n = 13 for Il27raf/f, n = 12 for Cd2-Cre Il27raf/f and n = 19 for Lyz2-Cre Il27raf/f) were performed after 10 weeks of HFD treatment. h. Adipose tissues were collected and weighted after 10 weeks of HFD treatment (n = 22 for Il27raf/f, n = 11 for Cd2-Cre Il27raf/f and n = 19 for Lyz2-Cre Il27raf/f). All experiments were repeated at least twice with similar results. Data are mean ± s.e.m. of biologically independent samples. Two-tailed unpaired student’s test (c, d); two-way ANOVA (e–g); one-way ANOVA (h). NS, not significant.
Extended Data Fig. 5 The phenotypes of IL-27Rα KO and WT chimeras in HFD-induced obesity and adaptive thermogenesis.
a. Bone marrow cells from CD45.1 WT strain were transferred into irradiated CD45.2 IL-27Rα KO or CD45.2 WT hosts (1 x 107cells/mouse) to generate chimera. Mice were housed for 8 weeks to reconstitute the immune system. Immune cells were isolated from different tissues as indicated, the percentages of donor (CD45.1+) and host (CD45.2+) cells in CD4+ T (top), CD19+ B (middle) or F4/80+ macrophages (bottom) were analysed via FACS (n = 3). b–d. Bone marrow chimeras were generated as in Fig. 3a and fed on HFD. GTT (b, n = 13 for WT>WT, n = 11 for WT>KO, n = 12 for KO>WT and n = 10 for KO>KO) and ITT (c, n = 5 for WT>WT, n = 7 for WT>KO, n = 6 for KO>WT and n = 6 for KO>KO) were performed after 10 weeks of HFD treatment. d. Epididymal adipose (n = 6 for WT>WT, n = 4 for the rest groups), subcutaneous adipose, brown adipose tissue and liver (n = 6 for WT>WT, n = 5 for WT>KO, n = 5 for KO>WT and n = 4 for KO>KO) were collected and weighted after 10 weeks of HFD treatment. e–g. Chimeric mice fed on normal chow were challenged at 4 °C for 12 hours, SCW and BAT were collected for histology analysis (e, H&E, scale bar = 100µm) or immunohistochemical staining of UCP1 (f, scale bar = 100µm). Real-time PCR analysis of gene expression in SCW was also performed and shown (g, in WT>WT group, n = 15 for Ucp1, n=16 for Cidea, Ppargc1a and Elovl3, n = 17 for Cox8b and Retn, n=18 for the rest genes; in WT>KO group, n = 11 for Ucp1, n = 12 for Cidea, Ppargc1a, Ppara and Lep, n=13 for Cox8b, Prdm16 and Retn, n = 14 for Cox5a and Fabp4, n = 15 for Elovl3, Pparg and Adipoq). All experiments were repeated at least twice with similar results. Data are mean ± s.e.m. of biologically independent samples. Two-tailed unpaired student’s t-test (a & g); one-way ANOVA (d); two-way ANOVA with Sidak’s multiple comparisons test (b & c).
Extended Data Fig. 6 Adipoq-Cre Il27raf/f mice were hypersensitive to HFD-induced obesity.
a. Immunoblot analysis of IL-27Rα expression in stromal vascular fraction (SVF) and adipocyte fraction of SCW and BAT from WT mice. b. Immunoblot analysis of IL-27Rα during the primary beige adipocyte differentiation from SVF of WT SCW. c. IL-27Rα expression in adipocyte fraction of eWAT / SCW or spleen from Il27raf/f and Adipoq-Cre Il27raf/f mice were determined by Real-time PCR (above; for Il27raf/f samples, n=6 for eWAT and spleen, n = 9 for SCW; for Adipoq-Cre Il27raf/f samples, n = 7 for eWAT, n = 10 for SCW and n = 5 for spleen) and Western Blot (below). d. Primary adipocytes were differentiated in vitro from SCW of Il27raf/f and Adipoq-Cre Il27raf/f mice. IL-27Rα protein expression was detected by immunofluorescence. IL-27Rα (Red), lipid droplet (Bodipy, Green) and cell nucleus (DAPI, Blue), scale bar = 20µm. e–l. Adipoq-Cre Il27raf/f mice and Il27raf/f controls at 8 weeks of age were fed on HFD for 10 weeks. e. Histology (H&E) staining of epididymal and subcutaneous fat tissue from HFD treated mice. Scale bar=100µm. GTT (f, n = 9 for Il27raf/f and n = 7 for Adipoq-Cre Il27raf/f), ITT (g, n=19 for Il27raf/f and n=14 for Adipoq-Cre Il27raf/f) and the weight of indicated tissues (h, n = 9 for Il27raf/f and n = 7 for Adipoq-Cre Il27raf/f) were detected after 10 weeks of HFD treatment. i. Histology (H&E) and oil red O staining of liver from HFD treated mice. Scale bar = 100µm. j. Serum cholesterol (CHOL, n = 8 for Il27raf/f and n = 7 for Adipoq-Cre Il27raf/f) and liver triglyceride (TG, n=9 for Il27raf/f and n=7 for Adipoq-Cre Il27raf/f) were detected. k. Realtime PCR analysis of inflammatory genes expression in eWAT (n = 9 for Il27raf/f samples, for Adipoq-Cre Il27raf/f group, n = 10 for Il12b, n=13 for Il6 and n = 12 for the rest genes). l. Realtime PCR analysis of adipose related genes expression in SCW of mice housed at room temperature (for Il27raf/f samples, n = 9 for Ucp1, n = 10 for Ppara, Elovl3 and Retn, n=12 for Cox5a and n = 11 for the rest genes; for Adipoq-Cre Il27raf/f group, n = 12 for Cox5a and Elovl3, n = 13 for the rest genes). m. Adipoq-Cre Il27raf/f and Il27raf/f mice fed on normal chow were challenged at 4 °C for 12 hours, SCW and BAT were collected for histology analysis (H&E). Scale bar = 50µm. All experiments were repeated at least twice with similar results. Data are mean ± s.e.m. of biologically independent samples. Two-tailed unpaired student’s t-test (c, h, j–l); two-way ANOVA with Sidak’s multiple comparisons test (f & g). NS, not significant.
Extended Data Fig. 7 IL-27 upregulates UCP1 and improves the browning of subcutaneous white adipose tissue.
a. Il27raf/f and Ucp1-Cre-ERT2 Il27raf/f mice at 8 weeks of age were i.p. injected with tamoxifen (2mg/mouse) for 4 times in a period of 7days, after another 7 days, SCWs and BATs were collected and used for immunoblot analysis of IL-27Rα expression. b&c. Il27raf/f (n=10) and Ucp1-Cre-ERT2 Il27raf/f (n = 12) mice at 8 weeks of age were pre-treated with tamoxifen and then fed on HFD for 10 weeks. GTT (b, n = 10 for Il27raf/f and n = 12 for Ucp1-Cre-ERT2 Il27raf/f) and ITT (c, n = 9 for Il27raf/f and n = 12 for Ucp1-Cre-ERT2 Il27raf/f) were performed after 10 weeks of HFD treatment. d. Il27raf/f and Ucp1-Cre-ERT2 Il27raf/f mice fed on normal chow were pre-treated with tamoxifen and then challenged at 4 °C for 12 hours, SCW and BAT were collected for histology analysis (H&E). Scale bar = 200µm. e. Primary beige adipocytes were generated in vitro from the SVF of WT SCW and then treated with rmIL-27 (100ng/ml) or PBS for 24 hours. Cells were lysed and protein extracts were used for Immunoblot analysis (each lane represents one biological independent sample). BAT tissue from WT mice was set as positive control. f. Immunoblot analysis of STAT1 phosphorylation in extracts from WT primary beige adipocytes treated with rmIL-27 (100ng/ml) for indicated time. IFN-γ (10ng/ml, 30 min) treated splenocytes were set as positive control (Con). g–h. Primary beige adipocytes from SCW of WT mice were generated in vitro. Two different p38 MAPK inhibitor (SB203580, 10µM and SB202190, 5µM) or three STAT3 inhibitor (C188-9, 10µM; stattic, 10µM or HO-3867, 20µM) were added into the culture medium 0.5 h before and in the duration of rmIL-27 treatment (100ng/ml for 12 h). The expression of UCP1 was detected via immunoblot. (e, g & h) Band densities were quantified with ImageJ and normalized. All experiments were repeated at least twice with similar results. Data are mean ± s.e.m. of biologically independent samples. Two-way ANOVA with Sidak’s multiple comparisons test (b & c).
Extended Data Fig. 8 IL-27 promotes the activation of thermogenesis with promising therapeutic potential.
a–i. Il27raf/f and Adipoq-Cre Il27raf/f mice were fed on HFD for 32 weeks and then i.p. injected with rmIL-27 (100µg/kg) or PBS every other day for 15 days. a. Serum were collected and used for detection of inflammatory factors by Bio-Rad Bio-Plex 200 Multiplexing Analyzer System (n = 8 for Il27raf/f + PBS, n=4 for Adipoq-Cre Il27raf/f + PBS and all IL-27 treatment groups). b. Representative histological sections (H&E) of indicated tissues, scale bar = 500µm. c. The percentages of the infiltrating CD4 or CD8 T cells, and the cytokines production in CD4 T cells from liver were analysed by FACS (n = 8 for Il27raf/f + PBS, n = 4 for Adipoq-Cre Il27raf/f + PBS, n = 5 for IL-27 treatment groups). Body weight (d), GTT (e) and ITT (f) of Il27raf/f mice were detected and recorded (n = 8 for PBS and n=5 for IL-27). Body weight (g), GTT (h) and ITT (i) of Adipoq-Cre Il27raf/f mice were also detected and recorded (n = 4 for PBS and n=5 for IL-27). j–l. Ucp1-Cre-ERT2 Il27raf/f mice were fed on HFD for 12 weeks, pretreated with tamoxifen and then i.p. injected with rmIL-27 (100µg/kg) or PBS every other day for 21 days. The body weight (j), glucose tolerance test (k) and insulin tolerance test (l) were detected and recorded (n = 5 for PBS and n = 6 for IL-27). m–p. UCP1 KO mice were fed on HFD for 20 weeks and then i.p. injected with rmIL-27 (100µg/kg) or PBS every other day for 15 days. m. Body weight was recorded at indicated time points (n=6). n. The adipose tissues and livers were collected and weighted after 15 days of rmIL-27 treatment (n = 6). GTT (o, n = 6) and ITT (p, n = 4) were performed after 15 days of rmIL-27 treatment. All experiments were repeated at least twice with similar results. Data are mean ± s.e.m. of biologically independent samples. One-way ANOVA (a & c), two-way ANOVA (d, g, j & m); two-way ANOVA with Sidak’s multiple comparisons test (e, f, h, i, k, l, o & p); two-tailed unpaired student’s t-test (n).
Extended Data Fig. 9 CX3CR1+ cells were an important source of IL-27 during HFD-induced obesity.
a–c. Lyz2-Cre Il27p28f/f and Il27p28f/f littermates at 8 weeks of age were treated with high fat diet (HFD) for 10 weeks. a. Body weight changes were recorded each week (n = 9 for Il27p28f/f and n = 6 for Lyz2-Cre Il27p28f/f). Intraperitoneal GTT (b) and ITT (c) were performed after 10 weeks of treatment (n = 7 for Il27p28f/f and n = 6 for Lyz2-Cre Il27p28f/f). d–g. Itgax-Cre Il27p28f/f and Il27p28f/f littermates at 8 weeks of age were fed on HFD for 10 weeks. Body weight changes were recorded each week (d, n = 8 for Il27p28f/f and n = 15 for Itgax-Cre Il27p28f/f). Intraperitoneal GTT (e, n = 8 for Il27p28f/f and n = 15 for Itgax-Cre Il27p28f/f) and ITT (f, n = 6) was performed after 10 weeks of treatment. g. Adipose tissues were collected and weighted after 10 weeks of HFD treatment (n = 8 for Il27p28f/f and n = 15 for Itgax-Cre Il27p28f/f). h&i. Adipoq-Cre Il27p28f/f and Il27p28f/f littermates at 8 weeks of age were fed on HFD for 10 weeks. h. Body weight was recorded each week (n = 6). i. Intraperitoneal GTT was performed after 10 weeks of treatment (n = 6). j–l. Cx3cr1-Cre Il27p28f/f and Il27p28f/f littermates at 8 weeks of age were fed on HFD for 10 weeks. j. Body weight was recorded each week (n = 8). Intraperitoneal GTT (k) and ITT (l) were performed after 10 weeks of treatment (n = 8). All experiments were repeated at least twice with similar results. Data are mean ± s.e.m. of biologically independent samples. Two-way ANOVA (a, d & h); two-way ANOVA with Sidak’s multiple comparisons test (b, c, e, f & i–l); two-tailed unpaired student’s t-test (g).
Supplementary information
Supplementary Figures
Supplementary Figure 1 contains the uncropped images of the western blot experiments and Supplementary Figure 2 shows the gating strategies for the FACS data in Extended Data Figure 2e.
Supplementary Table 1
Characteristics of human participants with obesity and control participants with a healthy BMI.
Supplementary Table 2
Characteristics of the individuals with type 2 diabetes in Extended Data Fig. 1c.
Supplementary Table 3
Characteristics of the human participants in Fig. 1b.
Supplementary Table 4
Gene set enrichment analysis of Il27ra-KO HFD versus WT HFD for Extended Data Fig. 3e, f.
Supplementary Table 5
Primer sequences for qPCR.
Source data
Rights and permissions
About this article
Cite this article
Wang, Q., Li, D., Cao, G. et al. IL-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature 600, 314–318 (2021). https://doi.org/10.1038/s41586-021-04127-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04127-5
This article is cited by
-
Enhancing insulin sensitivity in type 2 diabetes mellitus using apelin-loaded small extracellular vesicles from Wharton’s jelly-derived mesenchymal stem cells: a novel therapeutic approach
Diabetology & Metabolic Syndrome (2024)
-
Myeloid-derived grancalcin instigates obesity-induced insulin resistance and metabolic inflammation in male mice
Nature Communications (2024)
-
Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases
Signal Transduction and Targeted Therapy (2024)
-
Ginseng extracts improve circadian clock gene expression and reduce inflammation directly and indirectly through gut microbiota and PI3K signaling pathway
npj Biofilms and Microbiomes (2024)
-
Cytokimera GIL-11 rescued IL-6R deficient mice from partial hepatectomy-induced death by signaling via non-natural gp130:LIFR:IL-11R complexes
Communications Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.