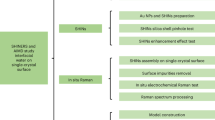
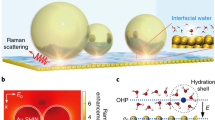
Abstract
Understanding the structure and dynamic process of water at the solid–liquid interface is an extremely important topic in surface science, energy science and catalysis1,2,3. As model catalysts, atomically flat single-crystal electrodes exhibit well-defined surface and electric field properties, and therefore may be used to elucidate the relationship between structure and electrocatalytic activity at the atomic level4,5. Hence, studying interfacial water behaviour on single-crystal surfaces provides a framework for understanding electrocatalysis6,7. However, interfacial water is notoriously difficult to probe owing to interference from bulk water and the complexity of interfacial environments8. Here, we use electrochemical, in situ Raman spectroscopic and computational techniques to investigate the interfacial water on atomically flat Pd single-crystal surfaces. Direct spectral evidence reveals that interfacial water consists of hydrogen-bonded and hydrated Na+ ion water. At hydrogen evolution reaction (HER) potentials, dynamic changes in the structure of interfacial water were observed from a random distribution to an ordered structure due to bias potential and Na+ ion cooperation. Structurally ordered interfacial water facilitated high-efficiency electron transfer across the interface, resulting in higher HER rates. The electrolytes and electrode surface effects on interfacial water were also probed and found to affect water structure. Therefore, through local cation tuning strategies, we anticipate that these results may be generalized to enable ordered interfacial water to improve electrocatalytic reaction rates.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data generated or analysed during this study are included in this published article and its Supplementary information files. Source data are provided with this paper.
Code availability
The code that supports the findings of this research is available from the corresponding authors upon reasonable request.
References
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Mubeen, S. et al. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotechnol. 8, 247–251 (2013).
Guo, J. et al. Real-space imaging of interfacial water with submolecular resolution. Nat. Mater. 13, 184−189 (2014).
Vidal-Iglesias, F. J., Solla-Gullon, J., Herrero, E., Aldaz, A. & Feliu, J. M. Pd adatom decorated (100) preferentially oriented Pt nanoparticles for formic acid electrooxidation. Angew. Chem. Int. Ed. 49, 6998–7001 (2010).
Strmcnik, D. et al. Enhanced electrocatalysis of the oxygen reduction reaction based on patterning of platinum surfaces with cyanide. Nat. Chem. 2, 880–885 (2010).
Kendrick, E., Kendrick, J., Knight, K. S., Islam, M. S. & Slater, P. R. Cooperative mechanisms of fast-ion conduction in gallium-based oxides with tetrahedral moieties. Nat. Mater. 6, 871–875 (2007).
Mesa, C. A. et al. Multihole water oxidation catalysis on haematite photoanodes revealed by operando spectroelectrochemistry and DFT. Nat. Chem. 12, 82–89 (2020).
Velasco-Velez, J. J. et al, The structure of interfacial water on gold electrodes studied by x-ray absorption spectroscopy. Science 346, 831–834 (2014).
Subbaraman, R. et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550–557 (2012).
Wang, X., Xu, C., Jaroniec, M., Zheng, Y. & Qiao, S. Z. Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions. Nat. Commun. 10, 4876 (2019).
Ledezma-Yanez, I. et al. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2, 17031 (2017).
Guha, A., Narayanaru, S. & Narayanan, T. N. Tuning the hydrogen evolution reaction on metals by lithium salt. ACS Appl. Energy Mater. 1, 7116–7122 (2018).
Guha, A., Kaley, N. M., Mondal, J. & Narayanan, T. N. Engineering the hydrogen evolution reaction of transition metals: effect of Li ions. J. Mater. Chem. A 8, 15795–15808 (2020).
Ataka, K., Yotsuyanagi, T. & Osawa, M. Potential-dependent reorientation of water molecules at an electrode/electrolyte interface studied by surface-enhanced infrared absorption spectroscopy. J. Phys. Chem. 100, 10664–10672, (1996).
Yamakata, A. & Osawa, M. Destruction of the hydration shell around tetraalkylammonium ions at the electrochemical interface. J. Am. Chem. Soc. 131, 6892–6893, (2009).
Tong, Y., Lapointe, F., Thamer, M., Wolf, M. & Campen, R. K. Hydrophobic water probed experimentally at the gold electrode/aqueous interface. Angew. Chem. Int. Ed. 56, 4211–4214 (2017).
Liu, W. T. & Shen, Y. R. In situ sum-frequency vibrational spectroscopy of electrochemical interfaces with surface plasmon resonance. Proc. Natl Acad. Sci. USA 111, 1293–1297 (2014).
Li, J. F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010).
Dong, J. C. et al. In situ Raman spectroscopic evidence for oxygen reduction reaction intermediates at platinum single-crystal surfaces. Nat. Energy 4, 60–67 (2019).
Davis, J. G., Gierszal, K. P., Wang, P. & Ben-Amotz, D. Water structural transformation at molecular hydrophobic interfaces. Nature 491, 582–585 (2012).
Chen, Y. X., Zou, S. Z., Huang, K. Q. & Tian, Z. Q. SERS studies of electrode/electrolyte interfacial water part II–librations of water correlated to hydrogen evolution reaction. J. Raman Spectrosc. 29, 749–756 (1998).
Toney, M. F. et al. Voltage-dependent ordering of water molecules at an electrode-electrolyte interface. Nature 368, 444–446 (1994).
Senior, W. A. & Thompson, W. K. Assignment of the infra-red and Raman bands of liquid water. Nature 205, 170 (1965).
Chen, H. C. et al. Active and stable liquid water innovatively prepared using resonantly illuminated gold nanoparticles. ACS Nano 8, 2704–2713 (2014).
Scatena, L. F., Brown, M. G. & Richmond, G. L. Water at hydrophobic surfaces: weak hydrogen bonding and strong orientation effects. Science 292, 908–912 (2001).
Lambert, D. K. Vibrational Stark effect of adsorbates at electrochemical interfaces. Electrochim. Acta 41, 623–630 (1996).
Li, J. F. et al. SERS and DFT study of water on metal cathodes of silver, gold and platinum nanoparticles. Phys. Chem. Chem. Phys. 12, 2493–2502 (2010).
Subbaraman, R. et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 334, 1256–1260 (2011).
Kibler, L. A. Hydrogen electrocatalysis. ChemPhysChem 7, 985–991 (2006).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Tonigold, K. & Gross, A. Dispersive interactions in water bilayers at metallic surfaces: a comparison of the PBE and RPBE functional including semiempirical dispersion corrections. J. Comput. Chem. 33, 695–701 (2012).
Sakong, S., Forster-Tonigold, K. & Gross, A. The structure of water at a Pt(111) electrode and the potential of zero charge studied from first principles. J. Chem. Phys. 144, 194701 (2016).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2019YFA0705400, 2020YFB1505800, 2020YFB0704500 and 2019YFD0901100), the National Natural Science Foundation of China (21925404, 22021001, 21521004, 21775127, 21991151 and 21902137), the Shenzhen Science and Technology Research Grant (JCYJ20200109140416788) and ‘111’ Project (B17027). We thank Y. X. Chen, B. W. Mao, D. P. Zhan, Z. Wei and J. B. Le for fruitful discussions, and Q. Q. Zhao, M. F. Cao and Y. Hui for technical assistance.
Author information
Authors and Affiliations
Contributions
Y.-H.W., S.Z., F.P. and J.-F.L. conceived the project; Y.-H.W., R.-Y.Z. and Q.-F.H. conducted the experiments; F.P., S.Z., S.L. and J.Z. performed the mechanism study and AIMD simulation; W.-M.Y. and Z.-L.Y. performed the 3D-FDTD simulation; Y.-H.W., S.Z., P.R., G.A., F.P., Z.-Q.T. and J.-F.L. wrote the paper. All authors participated in the analysis and discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Angel Cuesta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file includes Supplementary Notes, Figs. 1–21, Table 1 and Refs. 1–29.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, YH., Zheng, S., Yang, WM. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021). https://doi.org/10.1038/s41586-021-04068-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04068-z
This article is cited by
-
Bubble-water/catalyst triphase interface microenvironment accelerates photocatalytic OER via optimizing semi-hydrophobic OH radical
Nature Communications (2024)
-
Sustainable conversion of alkaline nitrate to ammonia at activities greater than 2 A cm−2
Nature Communications (2024)
-
RSPSSL: A novel high-fidelity Raman spectral preprocessing scheme to enhance biomedical applications and chemical resolution visualization
Light: Science & Applications (2024)
-
Complementary probes for the electrochemical interface
Nature Reviews Chemistry (2024)
-
Keto-anthraquinone covalent organic framework for H2O2 photosynthesis with oxygen and alkaline water
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.