Abstract
The availability of l-arginine in tumours is a key determinant of an efficient anti-tumour T cell response1,2,3,4. Consequently, increases of typically low l-arginine concentrations within the tumour may greatly potentiate the anti-tumour responses of immune checkpoint inhibitors, such as programmed death-ligand 1 (PD-L1)-blocking antibodies5. However, currently no means are available to locally increase intratumoural l-arginine levels. Here we used a synthetic biology approach to develop an engineered probiotic Escherichia coli Nissle 1917 strain that colonizes tumours and continuously converts ammonia, a metabolic waste product that accumulates in tumours6, to l-arginine. Colonization of tumours with these bacteria increased intratumoural l-arginine concentrations, increased the number of tumour-infiltrating T cells and had marked synergistic effects with PD-L1 blocking antibodies in the clearance of tumours. The anti-tumour effect of these bacteria was mediated by l-arginine and was dependent on T cells. These results show that engineered microbial therapies enable metabolic modulation of the tumour microenvironment leading to enhanced efficacy of immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD027167. Source data are provided with this paper.
References
Rodriguez, P. C. & Ochoa, A. C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev. 222, 180-191 (2008).
Bronte, V. & Zanovello, P. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5, 641-654 (2005).
Geiger, R. et al. l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829-842.e813 (2016).
Martí i Líndez, A.-A. M. et al. Mitochondrial arginase-2 is a cell‑autonomous regulator of CD8+ T cell function and antitumor efficacy. JCI Insight 4, e132975 (2019).
He, X., Lin, H., Yuan, L. & Li, B. Combination therapy with l-arginine and α-PD-L1 antibody boosts immune response against osteosarcoma in immunocompetent mice. Cancer Biol. Ther. 18, 94-100 (2017).
Spinelli, J. B. et al. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science 358, 941-946 (2017).
Forbes, N. S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 10, 785 (2010).
Kitada, T., DiAndreth, B., Teague, B. & Weiss, R. Programming gene and engineered-cell therapies with synthetic biology. Science 359, eaad1067 (2018).
Zhao, G., Jin, Z., Allewell, N. M., Tuchman, M. & Shi, D. Crystal structure of the N-acetyltransferase domain of human N-acetyl-l-glutamate synthase in complex with N-acetyl-l-glutamate provides insights into its catalytic and regulatory mechanisms. PLoS ONE 8, e70369 (2013).
Sancho-Vaello, E., Fernandez-Murga, M. L. & Rubio, V. Mechanism of arginine regulation of acetylglutamate synthase, the first enzyme of arginine synthesis. FEBS Lett. 583, 202-206 (2009).
Rajagopal, B. S., DePonte, J., 3rd, Tuchman, M. & Malamy, M. H. Use of inducible feedback-resistant N-acetylglutamate synthetase (argA) genes for enhanced arginine biosynthesis by genetically engineered Escherichia coli K-12 strains. Appl. Environ. Microbiol. 64, 1805-1811 (1998).
Aebersold, R. & Mann, M. Mass spectrometry-based proteomics. Nature 422, 198-207 (2003).
Malissen, M. et al. Altered T cell development in mice with a targeted mutation of the CD3‐epsilon gene. EMBO J. 14, 4641-4653 (1995).
Kurtz, C. B. et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med. 11, aau7975 (2019).
Isabella, V. M. et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat. Biotechnol. 36, 857-864 (2018).
Malissen, M. et al. Altered T cell development in mice with a targeted mutation of the CD3-epsilon gene. EMBO J. 14, 4641-4653 (1995).
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896-1906 (2007).
Scheltema, R. A. et al. The Q Exactive HF, a benchtop mass spectrometer with a pre-filter, high-performance quadrupole and an ultra-high-field Orbitrap analyzer. Mol. Cell. Proteomics 13, 3698-3708 (2014).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367-1372 (2008).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794-1805 (2011).
Acknowledgements
R.G. is supported in part by Synlogic, the European Research Council (803150) and Swiss Cancer Research (KFS-4593-08-2018). F.S. is supported by the Helmut Horten Foundation.
Author information
Authors and Affiliations
Contributions
R.G. and J.M.L. conceived the project. F.P.C., C.B., G.A., M.P., S.G., J.N. and W.J. performed experiments with mouse tumour models. N.L. and M.J.J. generated bacterial strains. J.-P.T. analysed tissue sections. A.S. and K.A.W. contributed to discussions. R.G., F.S. and J.M.L. supervised the work. D.S.L., J.M.L. and R.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.G. and J.L. are inventors on a patent application related to l-Arg bacteria. R.G. received research funding from Synlogic. N.L., A.S., M.J, D.S.L, K.A.W. and J.M.L. are or were employees and stockholders of Synlogic.
Additional information
Peer review information Nature thanks Jeff Hasty and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
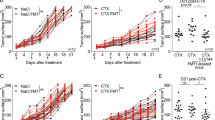
Extended Data Fig. 1 Intratumoral injections of an L-arginine solution does not affect the growth of MC38 tumors.
(a) MC38 tumors were established in C57BL/6 WT mice. Ten days later, 50 μl of a saturated L-arginine solution was injected into tumors. This corresponds to 0.25 mg L-arginine/g body weight and is the maximal volume that tumors can take up. Where indicated, mice were treated with 200µg anti-PD-L1 antibodies or isotype control antibodies by i.p injection. Mice received a total of four injections (bi-weekly). MC38 tumor growth curves. Values represent mean tumor volume ± s.e.m. Number of mice are indicated in the graph. Two experiments. (b) Same experiment as in (a) but survival curves are shown. (c) Same experiment as in (a) but growth curves of individual mice are shown. (d) Example of the gating strategy to quantify CD4 and CD8 T cells used in Figs. 3b, c, f, g
Extended Data Fig. 2 EcN colonization does not affect the growth of MC38 tumors.
MC38 tumors were established in C57BL/6 WT mice. Mice were treated with 5 x 106 CFU EcN (i.t.) or with i.t. injections of PBS, bi-weekly (four treatments in total). MC38 tumor growth curves. Values represent mean tumor volume ± s.e.m. Number of mice are indicated in the graph. Two experiments
Extended Data Fig. 3 The anti-tumor effect of L-Arg bacteria is T cell-dependent.
(a) MC38 tumors were established in C57BL/6 WT mice and in CD3e-/- mice and treated via intratumoral injection with 5 x 106 CFUs of L-Arg bacteria or EcN. Tumors were harvested and homogenized after 24 h, and bacterial abundance was measured by CFU assay. n = 2 (b) MC38 tumors were established in CD3e-/- mice. Tumors of the control group were treated with 5 x 106 CFUEcN (i.t.) twice a week. A second group was treated with 5 x 106 CFU L-Arg bacteria (i.t.) twice a week. A third group received anti-PD-L1 antibodies i.p. and 5 x 106 CFU EcN i.t. (EcN + α-PD-L1) and a fourth group received anti-PD-L1 antibodies i.p. and 5 x 106 L-Arg bacteria i.t (L-Arg-bac. + α-PD-L1). Tumor growth curve. Values represent mean tumor volume ± s.e.m. n = 5 for each group. (c) Survival curves of mice
Extended Data Fig. 4 Effect of bacterial treatments on mouse health.
(a) C57BL/6 WT mice with established MC38 tumors were treated with four i.t. injections of 5 x 106 EcN or L-Arg bacteria, or with PBS. The weight of mice was followed over time. Bars represent the SEM, throughout. (b) C57BL/6 WT mice with established MC38 tumors were treated with a single i.v. injection of 5 x 107 EcN or L-Arg bacteria, or with PBS. The weight of mice was followed over time. The number of mice is indicated in the graph. Two experiments (a, b)
Extended Data Fig. 5 Bacterial treatments and PD-L1 blockade have no effect on B16 tumor growth.
C57BL/6 WT mice with established B16.OVA tumors were treated four times with i.t. injections of 5 x 106 EcN or L-Arg bacteria and i.p. injections of anti-PD-L1 antibodies. Tumor growth curves. Values represent mean tumor volume ± s.e.m., n = 5
Supplementary information
Rights and permissions
About this article
Cite this article
Canale, F.P., Basso, C., Antonini, G. et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598, 662–666 (2021). https://doi.org/10.1038/s41586-021-04003-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04003-2
This article is cited by
-
FFAR2 expressing myeloid-derived suppressor cells drive cancer immunoevasion
Journal of Hematology & Oncology (2024)
-
Bacteria-driven nanosonosensitizer delivery system for enhanced breast cancer treatment through sonodynamic therapy-induced immunogenic cell death
Journal of Nanobiotechnology (2024)
-
HKDC1 promotes tumor immune evasion in hepatocellular carcinoma by coupling cytoskeleton to STAT1 activation and PD-L1 expression
Nature Communications (2024)
-
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2024)
-
Intratumoural microbiota: a new frontier in cancer development and therapy
Signal Transduction and Targeted Therapy (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.