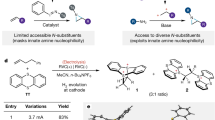
Abstract
Molecules that contain the β-arylethylamine motif have applications in the modulation of pain, treatment of neurological disorders and management of opioid addiction, among others, making it a privileged scaffold in drug discovery1,2. De novo methods for their assembly are reliant on transformations that convert a small class of feedstocks into the target compounds via time-consuming multistep syntheses3,4,5. Synthetic invention can drive the investigation of the chemical space around this scaffold to further expand its capabilities in biology6,7,8,9. Here we report the development of a dual catalysis platform that enables a multicomponent coupling of alkenes, aryl electrophiles and a simple nitrogen nucleophile, providing single-step access to synthetically versatile and functionally diverse β-arylethylamines. Driven by visible light, two discrete copper catalysts orchestrate aryl-radical formation and azido-group transfer, which underpin an alkene azidoarylation process. The process shows broad scope in alkene and aryl components and an azide anion performs a multifaceted role both as a nitrogen source and in mediating the redox-neutral dual catalysis via inner-sphere electron transfer10,11. The synthetic capabilities of this anion-mediated alkene functionalization process are likely to be of use in a variety of pharmaceutically relevant and wider synthetic applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Materials and methods, optimization studies, experimental procedures, mechanistic studies, 1H NMR, 13C NMR and 19F NMR spectra, and high-resolution mass spectrometry, infrared, ultraviolet–visible and cyclic voltammetry data are available in the Supplementary Information. Crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre under the reference numbers CCDC 2027142, CCDC 2027143 and CCDC 2032989. Raw data are available from the corresponding author on reasonable request.
References
Freeman, S. & Alder, J. F. Arylethylamine psychotropic recreational drugs: a chemical perspective. Eur. J. Med. Chem. 37, 527–539 (2002).
Dalley, J. W. & Everitt, B. J. Dopamine receptors in the learning, memory and drug reward circuitry. Semin. Cell Dev. Biol. 20, 403–410 (2009).
Barton, D. H. R., Bracho, R. D. & Widdowson, D. A. A new β-arylethylamine synthesis by aryl aldehyde homologation. J. Chem. Soc. Chem. Commun. 781a–781a (1973).
Schulze, M. Synthesis of 2-arylethylamines by the curtius rearrangement. Synth. Commun. 40, 1461–1476 (2010).
Bracher, F. Methods for arylethylation of amines and heteroarenes. SynOpen 02, 0096–0104 (2018).
Müller, T. E., Hultzsch, K. C., Yus, M., Foubelo, F. & Tada, M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem. Rev. 108, 3795–3892 (2008).
Nguyen, T. M., Manohar, N. & Nicewicz, D. A. Anti-Markovnikov hydroamination of alkenes catalyzed by a two-component organic photoredox system: direct access to phenethylamine derivatives. Angew. Chem. Int. Edn 53, 6198–6201 (2014).
Boyington, A. J., Seath, C. P., Zearfoss, A. M., Xu, Z. & Jui, N. T. Catalytic strategy for regioselective arylethylamine synthesis. J. Am. Chem. Soc. 141, 4147–4153 (2019).
Estrada, J. G., Williams, W. L., Ting, S. I. & Doyle, A. G. Role of electron-deficient olefin ligands in a Ni-catalyzed aziridine cross-coupling to generate quaternary carbons. J. Am. Chem. Soc. 142, 8928–8937 (2020).
Haim, A. Role of the bridging ligand in inner-sphere electron-transfer reactions. Acc. Chem. Res. 8, 264–272 (1975).
Rorabacher, D. B. Electron transfer by copper centers. Chem. Rev. 104, 651–698 (2004).
Zeng, W. & Chemler, S. R. Copper(II)-catalyzed enantioselective intramolecular carboamination of alkenes. J. Am. Chem. Soc. 129, 12948–12949 (2007).
Zhang, G., Cui, L., Wang, Y. & Zhang, L. Homogeneous gold-catalyzed oxidative carboheterofunctionalization of alkenes. J. Am. Chem. Soc. 132, 1474–1475 (2010).
Brenzovich, W. E. Jr et al. Gold-catalyzed intramolecular aminoarylation of alkenes: C–C bond formation through bimolecular reductive elimination. Angew. Chem. Int. Edn 49, 5519–5522 (2010).
Schultz, D. M. & Wolfe, J. P. Recent developments in palladium-catalyzed alkene aminoarylation reactions for the synthesis of nitrogen heterocycles. Synthesis 44, 351–361 (2012).
Yang, H.-B., Pathipati, S. R. & Selander, N. Nickel-catalyzed 1,2-aminoarylation of oxime ester-tethered alkenes with boronic acids. ACS Catal. 7, 8441–8445 (2017).
Lerchen, A., Knecht, T., Daniliuc, C. G. & Glorius, F. Unnatural amino acid synthesis enabled by the regioselective cobalt(III)-catalyzed intermolecular carboamination of alkenes. Angew. Chem. Int. Edn 55, 15166–15170 (2016).
Liu, Z. et al. Catalytic intermolecular carboamination of unactivated alkenes via directed aminopalladation. J. Am. Chem. Soc. 139, 11261–11270 (2017).
van der Puyl, V. A., Derosa, J. & Engle, K. M. Directed, nickel-catalyzed umpolung 1,2-carboamination of alkenyl carbonyl compounds. ACS Catal. 9, 224–229 (2019).
Jiang, H. & Studer, A. Intermolecular radical carboamination of alkenes. Chem. Soc. Rev. 49, 1790–1811 (2020).
Monos, T. M., McAtee, R. C. & Stephenson, C. R. J. Arylsulfonylacetamides as bifunctional reagents for alkene aminoarylation. Science 361, 1369–1373 (2018).
Wang, D. et al. Asymmetric copper-catalyzed intermolecular aminoarylation of styrenes: efficient access to optical 2,2-diarylethylamines. J. Am. Chem. Soc. 139, 6811–6814 (2017).
Heinrich, M. R., Blank, O. & Wölfel, S. Reductive carbodiazenylation of nonactivated olefins via aryl diazonium salts. Org. Lett. 8, 3323–3325 (2006).
Fumagalli, G., Boyd, S. & Greaney, M. F. Oxyarylation and aminoarylation of styrenes using photoredox catalysis. Org. Lett. 15, 4398–4401 (2013).
PrasadHari, D., Hering, T. & König, B. The photoredox-catalyzed meerwein addition reaction: intermolecular amino-arylation of alkenes. Angew. Chem. Int. Edn 53, 725–728 (2014).
Cativiela, C. & Díaz-de-Villegas, M. D. Recent progress on the stereoselective synthesis of acyclic quaternary α-amino acids. Tetrahedron Asymmetry 18, 569–623 (2007).
Venkatraman, J., Shankaramma, S. C. & Balaram, P. Design of folded peptides. Chem. Rev. 101, 3131–3152 (2001).
Hager, A., Vrielink, N., Hager, D., Lefranc, J. & Trauner, D. Synthetic approaches towards alkaloids bearing α-tertiary amines. Nat. Prod. Rep. 33, 491–522 (2016).
Clayden, J., Donnard, M., Lefranc, J. & Tetlow, D. J. Quaternary centres bearing nitrogen (α-tertiary amines) as products of molecular rearrangements. Chem. Commun. 47, 4624–4639 (2011).
Leggans, E. K., Barker, T. J., Duncan, K. K. & Boger, D. L. Iron(III)/NaBH4-mediated additions to unactivated alkenes: synthesis of novel 20′-vinblastine analogues. Org. Lett. 14, 1428–1431 (2012).
Huang, X., Bergsten, T. M. & Groves, J. T. Manganese-catalyzed late-stage aliphatic C–H azidation. J. Am. Chem. Soc. 137, 5300–5303 (2015).
Bunescu, A., Ha, T. M., Wang, Q. & Zhu, J. Copper-catalyzed three-component carboazidation of alkenes with acetonitrile and sodium azide. Angew. Chem. Int. Edn 56, 10555–10558 (2017).
Fu, N., Sauer, G. S., Saha, A., Loo, A. & Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 357, 575–579 (2017).
Heinrich, M. R. Intermolecular olefin functionalisation involving aryl radicals generated from arenediazonium salts. Chem. Eur. J. 15, 820–833 (2009).
Fischer, H. & Radom, L. Factors controlling the addition of carbon-centered radicals to alkenes—an experimental and theoretical perspective. Angew. Chem. Int. Edn 40, 1340–1371 (2001).
Odian, G.in Principles of Polymerization (ed. Odian, G.) 198–349 (Wiley, 2004); https://doi.org/10.1002/047147875X.ch3.
Tang, W. et al. Understanding atom transfer radical polymerization: effect of ligand and initiator structures on the equilibrium constants. J. Am. Chem. Soc. 130, 10702–10713 (2008).
Qiu, J., Matyjaszewski, K., Thouin, L. & Amatore, C. Cyclic voltammetric studies of copper complexes catalyzing atom transfer radical polymerization. Macromol. Chem. Phys. 201, 1625–1631 (2000).
Caldwell, J. J. et al. Identification of 4-(4-aminopiperidin-1-yl)-7H-pyrrolo[2,3-d]pyrimidines as selective inhibitors of protein kinase B through fragment elaboration. J. Med. Chem. 51, 2147–2157 (2008).
Addie, M. et al. Discovery of 4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide (AZD5363), an orally bioavailable, potent inhibitor of Akt kinases. J. Med. Chem. 56, 2059–2073 (2013).
Goel, P. et al. Recent advancement of piperidine moiety in treatment of cancer—a review. Eur. J. Med. Chem. 157, 480–502 (2018).
Suh, S.-E. et al. Site-selective copper-catalyzed azidation of benzylic C–H bonds. J. Am. Chem. Soc. 142, 11388–11393 (2020).
Alfassi, Z. B., Harriman, A., Huie, R. E., Mosseri, S. & Neta, P. The redox potential of the azide/azidyl couple. J. Phys. Chem. 91, 2120–2122 (1987).
Pavlishchuk, V. V. & Addison, A. W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorg. Chim. Acta 298, 97–102 (2000).
Bard, A. Standard Potentials in Aqueous Solution (Routledge, 1985).
Zhou, J. & Fu, G. C. Cross-couplings of unactivated secondary alkyl halides: room-temperature nickel-catalyzed Negishi reactions of alkyl bromides and iodides. J. Am. Chem. Soc. 125, 14726–14727 (2003).
Wang, Z., Yin, H. & Fu, G. C. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 563, 379–383 (2018).
Acknowledgements
We thank the Herchel Smith Fund at the University of Cambridge for a postdoctoral fellowship to A.B., the AstraZeneca–University of Cambridge PhD programme for studentship (Y.A.) and the Royal Society for Wolfson Merit Award to M.J.G.
Author information
Authors and Affiliations
Contributions
A.B. and M.J.G. conceived the project. A.B. and Y.A. conducted the experiments. A.B., Y.A. and M.J.G. analysed and interpreted the results. A.B., Y.A. and M.J.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Materials and Methods, Figs. 1–40, Tables 1–10 and Refs. 1–155.
Rights and permissions
About this article
Cite this article
Bunescu, A., Abdelhamid, Y. & Gaunt, M.J. Multicomponent alkene azidoarylation by anion-mediated dual catalysis. Nature 598, 597–603 (2021). https://doi.org/10.1038/s41586-021-03980-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03980-8
This article is cited by
-
A general alkene aminoarylation enabled by N-centred radical reactivity of sulfinamides
Nature Chemistry (2024)
-
Metal-free photocatalytic cross-electrophile coupling enables C1 homologation and alkylation of carboxylic acids with aldehydes
Nature Communications (2024)
-
Photochemical single-step synthesis of β-amino acid derivatives from alkenes and (hetero)arenes
Nature Chemistry (2022)
-
Recent advances in visible light-induced C(sp3)–N bond formation
Nature Reviews Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.