Abstract
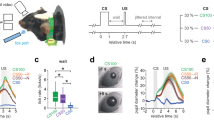
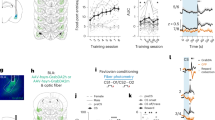
Mounting evidence shows that dopamine in the striatum is critically involved in reward-based reinforcement learning1,2. However, it remains unclear how dopamine reward signals influence the entorhinal–hippocampal circuit, another brain network that is crucial for learning and memory3,4,5. Here, using cell-type-specific electrophysiological recording6, we show that dopamine signals from the ventral tegmental area and substantia nigra control the encoding of cue–reward association rules in layer 2a fan cells of the lateral entorhinal cortex (LEC). When mice learned novel olfactory cue–reward associations using a pre-learned association rule, spike representations of LEC fan cells grouped newly learned rewarded cues with a pre-learned rewarded cue, but separated them from a pre-learned unrewarded cue. Optogenetic inhibition of fan cells impaired the learning of new associations while sparing the retrieval of pre-learned memory. Using fibre photometry, we found that dopamine sends novelty-induced reward expectation signals to the LEC. Inhibition of LEC dopamine signals disrupted the associative encoding of fan cells and impaired learning performance. These results suggest that LEC fan cells represent a cognitive map of abstract task rules, and that LEC dopamine facilitates the incorporation of new memories into this map.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Neurophysiological data generated in this study are available upon request, and will be deposited with a subsequent protocol paper.
References
Schultz, W., Dayan, P. & Montague, P. R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Watabe-Uchida, M., Eshel, N. & Uchida, N. Neural circuitry of reward prediction error. Annu. Rev. Neurosci. 40, 373–394 (2017).
Scoville, W. B. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957).
Squire, L. R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99, 195–231 (1992).
Buzsaki, G. & Moser, E. I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 16, 130–138 (2013).
Cohen, J. Y., Haesler, S., Vong, L., Lowell, B. B. & Uchida, N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88 (2012).
Witter, M. P. & Amaral, D. G. in The Rat Nervous System 3rd edn (ed. Paxinos, G.) Ch. 21 (Elsevier, 2004).
Moser, E. I. et al. Grid cells and cortical representation. Nat. Rev. Neurosci. 15, 466–481 (2014).
Igarashi, K. M., Lu, L., Colgin, L. L., Moser, M. B. & Moser, E. I. Coordination of entorhinal–hippocampal ensemble activity during associative learning. Nature 510, 143–147 (2014).
Kitamura, T. et al. Island cells control temporal association memory. Science 343, 896–901 (2014).
Surmeli, G. et al. Molecularly defined circuitry reveals input–output segregation in deep layers of the medial entorhinal cortex. Neuron 88, 1040–1053 (2015).
Vandrey, B. et al. Fan cells in layer 2 of the lateral entorhinal cortex are critical for episodic-like memory. Curr. Biol. 30, 169–175 (2020).
Leitner, F. C. et al. Spatially segregated feedforward and feedback neurons support differential odor processing in the lateral entorhinal cortex. Nat. Neurosci. 19, 935–944 (2016).
Ohara, S. et al. Entorhinal layer II calbindin-expressing neurons originate widespread telencephalic and intrinsic projections. Front. Syst. Neurosci. 13, 54 (2019).
Chuong, A. S. et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129 (2014).
Hokfelt, T., Ljungdahl, A., Fuxe, K. & Johansson, O. Dopamine nerve terminals in the rat limbic cortex: aspects of the dopamine hypothesis of schizophrenia. Science 184, 177–179 (1974).
Fallon, J. H., Koziell, D. A. & Moore, R. Y. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J. Comp. Neurol. 180, 509–532 (1978).
Swanson, L. W., Köhler, C. & Björklund, A. in Handbook of Chemical Neuroanatomy Vol. 5, Part I (eds Björklund, A., Hökfelt, T. & Swanson, L. W.) 125–227 (Elsevier, 1987).
Kudo, Y. et al. A single optical fiber fluorometric device for measurement of intracellular Ca2+ concentration: its application to hippocampal neurons in vitro and in vivo. Neuroscience 50, 619–625 (1992).
Menegas, W., Babayan, B. M., Uchida, N. & Watabe-Uchida, M. Opposite initialization to novel cues in dopamine signaling in ventral and posterior striatum in mice. Elife 6, e21886 (2017).
Igarashi, K. M. et al. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J. Neurosci. 32, 7970–7985 (2012).
Nilssen, E. S. et al. Inhibitory connectivity dominates the fan cell network in layer II of lateral entorhinal cortex. J. Neurosci. 38, 9712–9727 (2018).
Yagishita, S. et al. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345, 1616–1620 (2014).
Tse, D. et al. Schemas and memory consolidation. Science 316, 76–82 (2007).
Tolman, E. C. Cognitive maps in rats and men. Psychol. Rev. 55, 189–208 (1948).
Behrens, T. E. J. et al. What is a cognitive map? organizing knowledge for flexible behavior. Neuron 100, 490–509 (2018).
McNaughton, B. L., Battaglia, F. P., Jensen, O., Moser, E. I. & Moser, M. B. Path integration and the neural basis of the ‘cognitive map’. Nat. Rev. Neurosci. 7, 663–678 (2006).
Kakade, S. & Dayan, P. Dopamine: generalization and bonuses. Neural Netw. 15, 549–559 (2002).
Lisman, J. E. & Grace, A. A. The hippocampal–VTA loop: controlling the entry of information into long-term memory. Neuron 46, 703–713 (2005).
Takeuchi, T. et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362 (2016).
Mori, K., Takahashi, Y. K., Igarashi, K. M. & Yamaguchi, M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol. Rev. 86, 409–433 (2006).
Uchida, N. & Mainen, Z. F. Speed and accuracy of olfactory discrimination in the rat. Nat. Neurosci. 6, 1224–1229 (2003).
Li, Y. et al. (2017). A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat. Neurosci. 20, 559–570.
Kvitsiani, D. et al. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 20, 363–366 (2013).
Cunningham, J. P. & Byron, M. Y. Dimensionality reduction for large-scale neural recordings. Nat. Neurosci. 17, 1500–1509 (2014).
Russo, A. A. et al. Neural trajectories in the supplementary motor area and motor cortex exhibit distinct geometries, compatible with different classes of computation. Neuron 107, 745–758 (2020).
Sano, H. et al. (2003). Conditional ablation of striatal neuronal types containing dopamine D2 receptor disturbs coordination of basal ganglia function. J. Neurosci. 23, 9078–9088.
Acknowledgements
We thank N. Uchida and M. Watabe-Uchida for their mentorship, including support in setting up optogenetic recording and photometry experiments and providing discussions throughout the course of this project; R. Amo and H. Matsumoto in the Uchida laboratory for their help with setting up the behaviour and photometry experiments; T. Viaclovsky, A. Bramian and M. Savadkohighodjanaki in the Igarashi laboratory for technical assistance; and M. P. Witter, A. Treves, H. T. Ito, B. McNaughton, C. Gall and G. Lynch for providing comments on the work. The work was supported by NIH R01 grants (R01MH121736, R01AG063864, R01AG066806), a PRESTO grant from Japan Science and Technology Agency (JPMJPR1681), a Brain Research Foundation Fay-Frank Seed Grant (BRFSG-2017-04), a Whitehall Foundation Research Grant (2017-08-01), a BrightFocus Foundation Research grant (A2019380S), an Alzheimer’s Association Research Grant (AARG-17-532932) and a New Vision Research Investigator Award (CCAD201902) to K.M.I. H.J. was supported by the University of California, Irvine Medical Scientist Training Program (MSTP) (T32GM008620) and an NIH F31 grant (1F31AG069500).
Author information
Authors and Affiliations
Contributions
K.M.I. conceived the project and designed the experiments. J.Y.L., H.J., S.S., K.M.I., T. Nakazono, K.S., T. Nakagawa, J.L.X., J.C., R.R. and S.Y. performed the behaviour experiments. J.Y.L., H.J., S.S., K.M.I. and T. Nakazono performed the electrophysiology experiments. K.M.I. performed the pharmacology experiment. K.M.I. and A.D. performed the photometry experiment. J.Y.L., H.J. and S.S. performed the histology experiments. M.H. and K.M. performed in situ hybridization experiments. K.M.I., S.S., J.Y.L. and H.J. performed the analyses. K.M.I wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Histological validation of implanted sites.
(a). Optic fibre positions in the LEC of Sim1-Cre mice injected with AAV-flex-Jaws-GFP for inhibition experiments. Arrowhead, the tip of optic fibres. D, dorsal, V, ventral, M, medial, L, lateral. (b). Optic fibre positions in the LEC of Wfs1-Cre mice for inhibition experiment of Wfs1-expresing pyramidal cells. (c). Recording position in the superficial layer of LEC from Sim1-Cre mice for opt-tagging experiment. Note large lesions because of the electrical lesioning. (d). Optic fibre positions in the LEC of DAT-Cre mice injected with AAV-flex-Jaws-GFP in the VTA/SNc for inhibition experiment. (e). Optic fibre positions in the LEC of DAT-Cre x Ai14 mice for photometry experiment. Two mice received unilateral implantations and four mice received bilateral implantations. (f). Recording position in the superficial layer of LEC from Sim1-Cre x DAT-Cre mice for opt-tagging + inhibition experiment. (g). Optic fibre positions in the LEC of Sim1-Cre mice injected with AAV-flex-GFP for control inhibition experiment. (h). Optic fibre positions in the LEC of Wfs1-Cre mice injected with AAV-flex-GFP for control inhibition experiment. (i). Optic fibre positions in the LEC of DAT-Cre mice injected with AAV-flex-GFP at VTA and SNc for control inhibition experiment. (j). Recording position in the superficial layer of LEC from Sim1-Cre x DAT-Cre mice for opt-tagging (ChR2-mCherry) + control inhibition (GFP) experiment.
Extended Data Fig. 2 Performance of mice during associative learning.
(a). A model for LEC dopamine and fan cells in associative learning. When novel cues are presented, LEC dopamine functions as a “detonator” of fan cell activity. Dopamine gradually supplies reward expectation signals only during rewarded Odour A and Odour 1 trials, serving as a supervising signal so that Odour A and Odour 1 are represented in the same fan cell population. Odour B is represented by a fan cell population distinct from Odours-A/1, resulting in A-B separation. Novel unrewarded Odour 2 was not clearly represented in our recorded population. Two types of errors were observed in our study: The spontaneous error where A-B separation and A-1 overlap were both abolished (Fig. 2g), and the error observed in the unilateral dopamine inhibition sessions where A-B separation was spared but A-1 overlap was abolished (Fig. 4c). (b). SIM1 and WFS1 population counts in the LEC. (Top) Reelin immunohistochemistry in Sim1-Cre mice injected with AAV-DIO-mCherry. D, dorsal, V, ventral, M, medial, L, lateral. (Middle) Calbindin immunohistochemistry in Wfs1-Cre mice injected with AAV-DIO-mCherry. (Bottom) From left, density of mCherry-positive neurons in Sim1-Cre and Wfs1-Cre mice, Reelin and Calbindin positive cells, and percentage of mCherry-labelled neurons. (p > 0.05, Wilcoxon rank sum test; n = 16 sections obtained from n = 2 mice for each group). (c). We performed an additional experiment to inhibit fan cells during the whole period of pre-learning with Odour A and Odour B (these mice are not included in the data in the main figures). After injecting Jaws in Sim1-Cre mice, A/B training was repeated (n = 6 GFP control mice and n = 6 Jaws inhibition mice). Days until mice reached criterion (three consecutive days of reaching 80%) were compared (p = 0.012, Wilcoxon rank sum test). (d). Detailed performance of mice during fan cell inhibition. (d1) Learning curves during control (left) and fan cell inhibition (middle) sessions. In these plots, the data shown in Fig. 1d were plotted for percent correct trials in each odour trial type as a function of trial number for each odour type. (Right) Performance of mice in the last 10 trials (p = 6.1e-19, ANOVA; p = 6.0e-8, post-hoc Tukey test; n = 10 mice).(d2) Performance of mice in trials 121-160 as in Fig. 1d, but assessed with discriminability index (D-prime) (p=3.1e-3, ANOVA;p = 1.2e-5 or better, post-hoc Tukey test; n = 10 mice).(d3) We performed control experiment using Sim1-Cre mice injected with AAV-flex-GFP with laser (n = 5 mice). The GFP control experiment showed same result as no-laser control in Fig. 1d (p = 0.0028, ANOVA; p = 3.1e-5 or better, post-hoc Tukey test; n = 5 GFP control mice; n = 10 Jaws inhibition mice). (e). Same as (d), but for pyramidal cell inhibition in Wfs1-Cre mice in Fig. 2e (p > 0.05, ANOVA; n = 5 GFP control mice; n = 9 Jaws inhibition mice). (g). Same as (d), but for bilateral dopamine inhibition in DAT-Cre mice in Fig. 3e (p=0.0017, ANOVA; p = 2.0e-5 or better, post-hoc Tukey test; n = 4 GFP control mice; n = 9 Jaws inhibition mice). (f). Difference of percent correct sessions between control and inhibition as in Fig. 1f, but plotted for discriminability index (D-prime) (p = 0.019, ANOVA; p = 0.0021 or better, post-hoc Tukey test).
Extended Data Fig. 3 Performance and spikes in Sim1-Cre mice.
a, Behavioural performance of mice used for fan cell opt-tag recording (Fig. 2). (a1) Learning curves for Odour A/Odour B (blue) and Odour 1/Odour 2 (red) during correct sessions where mice acquired the association of Odours1/2 (Correct sessions, top left), and during error sessions where mice did not acquire the new association (Error sessions, top right). (Bottom left) Percent correct trials averaged for trials 121-160 for Odour A/B trials and Odour 1/2 trials during correct and error sessions (p=6.5e-5, ANOVA;p = 1.7e-6 or better, post-hoc Tukey test; n = 10 mice). (Bottom right) Percent of correct and error sessions. (a2). Learning curves during correct (left) and error (middle) sessions. In these plots, the data shown in (a1) were plotted for percent correct trials in each odour trial type as a function of trial number for each odour type. (Right) Performance of mice in the last 10 trials (p=3.3e-8, ANOVA;p = 0.001 or better, post-hoc Tukey test; n = 10 mice). (a3). Performance of mice in trials 121-160 as in (a1), but assessed with discriminability index (D-prime) (p=1.5e-4, ANOVA;p = 6.4e-6 or better, post-hoc Tukey test; n = 10 mice). b, A representative fan cell shown in Fig. 2b. (Right) Firing frequency in Trials 1 – 10 was plotted. Mean firing frequencies during 0.5 – 1.5 s after cue onset in each trial are shown in the bar graph. c-e, Three additional example fan cells that fired to Odour 1. These cells showed high firing frequency to Odour 1 within 10 trials. f, Mean firing frequency to each odour in trials 1 – 10 in T1. Fan cells showed larger firing frequency to Odour 1 than to other odours starting from trial 2 (n = 213 cells,p = 4.1e-30, ANOVA; p < 0.05 or better, post-hoc Tukey test).
Extended Data Fig. 4 Spike properties of fan cells in Sim1-Cre mice.
(a)–(c), spike properties of fan cells. Fan cells were recorded in a session with Odour A and Odour B (AB session). After ~20 trials in AB session, associative learning (AB12) session was tested (T1-T5). T5 in error sessions is also shown. (a) Spike firing of 213 fan cells. Mean spike activity was averaged in 50 ms bins and shown in z-score compared with −1 – 0 s before odour onset. In this panel, cells were sorted using a cluster analysis of firing property in T5. (b). Mean firing rate of 213 fan cells shown in z-score. (c) Percent responsive cells in periods of 0.5-1.5 s (odour), 2-3 s (delay) and 3-4 s (choice) after odour onset. Neurons with significant firing during each period were counted (Wilcoxon signed-rank test, p < 0.05). (d) Percent responsive cells in correct T5 (top) and error T5 (bottom). Neurons with significant firing during 0.5-1.5 s after odour onset were counted (Wilcoxon signed-rank test, p < 0.05). Asterisk denotes lower percentage of A-1 responsive cells in error T5 than that in correct T5 (p < 0.05, chi-square test; p < 0.05 for A-1 cells, post-hoc residual test with false discovery rate correction for multiple comparisons). (e)Trajectories of neural firing of fan cell population (top), Euclidian distance between odour trial types (middle) and mean Euclidian distance and similarity index during 0.5-1.5 s after odour onset (cue period) for timepoints T1 – T5 of correct sessions (bottom). Ninety-fifth percentile distance obtained from shuffled data denotes significant distance (red line). Data during 2-3 s (delay) and 3-4 s (choice) after odour onset were also plotted. (f). Same as (e), but for error sessions where mice did not learn new associations. (g). Example trajectories obtained from shuffling analysis. Trajectories of neuronal data obtained from three shuffled data in correct T5 sessions are shown. (h). Distribution of mean Euclidian distance obtained from shuffle data in correct T5. Distance obtained from six possible odour pairs were averaged and plotted. A 95th percentile of the distribution (red) was used for the cut-off indicating significant distance.
Extended Data Fig. 5 Bootstrapping test for spike similarity index.
The change of similarity index (SI) during associative learning was compared using the bootstrapping method. PCA was performed from a resampled neuronal population, and this procedure was repeated 1,000 times to make 1,000 bootstraps. SI was calculated for each bootstrap, then SIs in T2 – T5 were subtracted by that in T1, to test if there was a significant distribution above or below zero. (a). (Top) In correct sessions in Fig. 2, SI for Odours A-B showed significant decrease in T5 compared to T1 (p = 0.039) whereas SI for Odours A-1 increased (p = 1.2e-10), confirming A-B separation and A-1 overlap. (Middle) In the error sessions, no A-B separation was observed (p > 0.05). Although A-1 distance decreased during the session (p < 0.05), SIAB stayed in negative values. (Bottom) The subtraction of bootstraps in error session from correct sessions confirms the difference in A-1 overlap (p < 0.05 in T3 and T5, right). *p < 0.05, **p < 0.01, ***p < 0.001; n = 1,000, bootstrapping test. (b). (Top) In dopamine control sessions, SI for Odours A-B showed significant decrease in T5 compared to T1 (p = 0.044) whereas SI for Odours A-1 increased (p =0.0082), confirming A-B separation and A-1 overlap. (Middle) In the unilateral inhibition sessions, although A-B separation was observed (p < 0.05), no A-1 overlap was observed (p > 0.05). (Bottom) The subtraction of bootstraps in inhibition session from control sessions confirms the effect of inhibition on A-1 overlap (p < 0.05 in T3 – T5, right). These data suggest that dopamine plays a critical role in establishing A-1 overlapped representations. *p < 0.05, **p < 0.01, ***p < 0.001; n = 1,000, bootstrapping test.
Extended Data Fig. 6 Additional principal component analyses.
(a). Trajectories of neural firing of fan cell population using only correct (hit) trials for Odour A and Odour 1 and correct rejection (CR) trials for Odour B and Odour 2. The separation and overlap of fan cells were again observed when the incorrect trials were removed from the PC analysis. (b). Trajectories of neural firing of fan cell population using only hit trials for Odour A and Odour 1 and error lick (false alarm, FA) trials for Odour B and Odour 2. Although all of them are trials in which mice made lick responses, similar overlapped representations between Odours-A and 1, and their separation from Odour B were observed, suggesting that fan cells do not simply represent lick-related motor information. (c). Principal component analysis (PCA) for 213 fan cells as in Fig. 2d, but using conjunctive PCA with data from all timepoints (AB-only, T1 – T5). The results show similar A-1 overlap and A-B separation as in Fig. 2d.
Extended Data Fig. 7 Repeated associative learning using the same odour cues.
(a). To test the effect of repeated exposure to the same odour pair during associative learning, sessions with Odour A, -B, -C and -D were repeated for 10 days in Sim1xDAT mice injected with AAV-flex-GFP (n = 4 mice, without laser). (b). Learning curves between Day 1 – Day 4 and Day 5 – Day 10. In Day 1 – Day 4, mice gradually learned Odour C and Odour D as in the regular new association experiment with novel odour pairs. However, after Day 5, mice showed better performance for Odour C and Odour D from the initial trials. This was confirmed with increased correct rate for Odour C/D on trials 1 – 20 in Day 5 – 10 compared to that in Day 1 – 4 (bottom left,p = 3.5e-4, ANOVA with post-hoc Tukey test, p = 0.0025). (c). Fan cell trajectories for Odour A, -B, -C and -D in T5 during Day 1 – Day 4 (left, n = 101 cells) and during Day 5 – 10 (right, n = 93 cells). (d). Fan cells showed A-B separation and A-1 overlap in Day 1-4, but this representation disappeared after mice were overtrained. These results further support the idea that fan cells were needed only when new associative memory is formed.
Extended Data Fig. 8 Properties of LEC dopamine inputs.
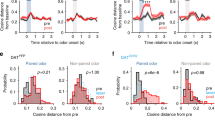
a-d, Pharmacological blockade experiments during associative learning. We performed a supplementary pharmacological experiment to validate the optogenetic inhibition experiments using dopamine D1 receptor antagonist SCH23390, or GABAA receptor agonist muscimol. SCH23390 bilateral injection abolished new learning of Odour 1 and Odour 2, while sparing the pre-learned association, replicating the result obtained from the optogenetic inhibition of dopamine fibres (Fig. 3e). Injection of muscimol impaired both the pre-learned association and acquisition of new association, implying an involvement of LEC neurons other than fan cells in the retrieval of pre-learned association. (a). Learning curves during saline, SCH23390 and muscimol infusions. (b). Percent correct sessions during trials 121 – 160 where mice correctly learned new association (p=6.1e-4, ANOVA; 0.0032 or better, post-hoc Tukey test; n = 5 mice). (c). Example histology from cannula implantations. (d). Learning curves during saline (left), SCH23390 (middle) and muscimol (right) sessions. In these plots, the data shown in (a) were plotted for percent correct trials in each odour trial type as a function of trial number for each odour type. (Right) Performance of mice in the last 10 trials (p=1.3e-15, ANOVA; p = 2.2e-7, post-hoc Tukey test; n = 5 mice). e-h, Retrograde tracing of LEC dopaminergic fibres from VTA and SNc. (e). Coronal section of the right hemisphere including LEC, where the retrograde tracer cholera toxin B (CTB, red) was injected. (f). Coronal section of the right hemisphere midbrain including VTA and SNc. Anti-tyrosine hydroxylase (TH, green) immunostaining reveals dopaminergic cells. (g). Magnified windows from (f). Yellow arrows point to example cells expressing both TH and CTB, which are further magnified in the rightmost panels. (h). From left, density of TH-expressing neurons in VTA and SNc, TH+CTB+ population between VTA and SNc, percentage of double-positive neurons among TH+ neurons. Although VTA has more cells for both TH+ and TH+CTB+ neurons (p < 0.001, Wilcoxon rank sum test), the percentage of CTB+ neurons did not differ between VTA and SNc (p=0.50, Wilcoxon rank sum test; n = 22 sections obtained from n = 3 mice). i-k, Calcium imaging of dopamine inputs. (i). Calcium signals from individual hemisphere (n = 10) during first 10 trials (T1, top) and last 10 trials (middle, T5) in correct sessions. Mean traces are shown at the bottom for T1 (black) and T5 (red). *p < 0.05 and **p < 0.01, Wilcoxon signed-rank test during 0.5 – 3 s after cue onset compared with 1-s pre-cue period. (j). Same as (b), but for error sessions. (k). Plot of GCaMP calcium signal as a function of trial number after starting AB12 session (n = 10 hemispheres).
Extended Data Fig. 9 Dopamine unilateral inhibition during fan cell recording.
(a) Performance of mice during unilateral dopamine inhibition. (Left) Performance of mice in trials 121-160 as in Fig. 4a, but assessed with discriminability index (D-prime) (p=5.1e-4, ANOVA;p = 9.6e-3 or better, post-hoc Tukey test; n = 8 mice). (Middle) Learning curves during control (left) and unilateral dopamine inhibition (right) sessions. In these plots, the data shown in Fig. 4a were plotted for percent correct trials in each odour trial type as a function of trial number for each odour type. Plot using 2-trial moving window is also shown for control sessions. (Right) Performance of mice in the last 10 trials (p=6.5e-6, ANOVA;p = 0.046 or better, post-hoc Tukey test; n = 10 mice). In the unilateral dopamine inhibition experiments, each mouse (n = 8) had 10 – 16 inhibition sessions. Of them, percentage of correct sessions (i.e. #correct sessions/(#correct sessions + #error sessions)) were 46.6%, 55.6%, 43.3%, 50.0%, 54.7%, 53.6%, 53.6%, and 42.8%. (b). We performed control experiments using DAT-Cre mice injected with AAV-DIO-ChR2 and AAV-flex-GFP for unilateral opt-tagging and laser control (n = 4 mice). The GFP control experiment showed same result as no-laser control in Fig. 4a (p=0.035, ANOVA;p = 0.044 or better, post-hoc Tukey test; n = 4 GFP control mice; n = 8 Jaws inhibition mice).
Extended Data Fig. 10 Firing property of fan cells during dopamine unilateral inhibition.
(a). Firing property of fan cells in the no-laser control sessions obtained from Sim1xDAT mice (n = 148 cells). (Top to bottom) Z-scored firing rates, mean firing rate, percent cells for each response type, PCA trajectories, Euclidian distance and mean Euclidian distance are shown as in Fig. 2d. (b). Same as (a), but for fan cells during unilateral dopamine inhibition (n = 134 cells). Mean firing rates for Odour A and Odour 1 were lower than control in T1 (p < 0.05, Wilcoxon rank sum test). No difference was observed for the distribution of responsive type in T5 between control and inhibition (p=0.24, chi-square test). (c). Trajectories, mean Euclidian distance and similarity index of fan cells as in Fig. 4b, but from GFP control mice (n = 130 cells).
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1–4.
Rights and permissions
About this article
Cite this article
Lee, J.Y., Jun, H., Soma, S. et al. Dopamine facilitates associative memory encoding in the entorhinal cortex. Nature 598, 321–326 (2021). https://doi.org/10.1038/s41586-021-03948-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03948-8
This article is cited by
-
A multiple hits hypothesis for memory dysfunction in Parkinson disease
Nature Reviews Neurology (2024)
-
Non-canonical interplay between glutamatergic NMDA and dopamine receptors shapes synaptogenesis
Nature Communications (2024)
-
Lateral entorhinal cortex subpopulations represent experiential epochs surrounding reward
Nature Neuroscience (2024)
-
Optogenetic activation of dopamine D1 receptors in island cells of medial entorhinal cortex inhibits temporal association learning
Molecular Brain (2023)
-
A novel micro-ECoG recording method for recording multisensory neural activity from the parietal to temporal cortices in mice
Molecular Brain (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.