Abstract
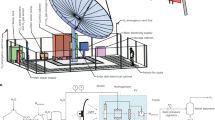
The unprecedented impact of human activity on Earth’s climate and the ongoing increase in global energy demand have made the development of carbon-neutral energy sources ever more important. Hydrogen is an attractive and versatile energy carrier (and important and widely used chemical) obtainable from water through photocatalysis using sunlight, and through electrolysis driven by solar or wind energy1,2. The most efficient solar hydrogen production schemes, which couple solar cells to electrolysis systems, reach solar-to-hydrogen (STH) energy conversion efficiencies of 30% at a laboratory scale3. Photocatalytic water splitting reaches notably lower conversion efficiencies of only around 1%, but the system design is much simpler and cheaper and more amenable to scale-up1,2—provided the moist, stoichiometric hydrogen and oxygen product mixture can be handled safely in a field environment and the hydrogen recovered. Extending our earlier demonstration of a 1-m2 panel reactor system based on a modified, aluminium-doped strontium titanate particulate photocatalyst4, we here report safe operation of a 100-m2 array of panel reactors over several months with autonomous recovery of hydrogen from the moist gas product mixture using a commercial polyimide membrane5. The system, optimized for safety and durability, and remaining undamaged on intentional ignition of recovered hydrogen, reaches a maximum STH of 0.76%. While the hydrogen production is inefficient and energy negative overall, our findings demonstrate that safe, large-scale photocatalytic water splitting, and gas collection and separation are possible. To make the technology economically viable and practically useful, essential next steps are reactor and process optimization to substantially reduce costs and improve STH efficiency, photocatalyst stability and gas separation efficiency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
References
Hisatomi, T. & Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor. Nat. Catal. 2, 387–399 (2019).
Kim, J. H., Hansora, D., Sharma, P., Jang, J.-W. & Lee, J. Toward practical solar hydrogen production – an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 48, 1908–1971 (2019).
Jia, J. et al. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 7, 13237 (2016).
Goto, Y. et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen production. Joule 2, 509–520 (2018).
Tanihara, N., Nakanishi, S. & Yoshinaga, T. Gas and vapor separation through polyimide membranes. J. Jpn. Petrol. Inst. 59, 276–282 (2016).
Pagliaro, M. Preparing for the future: solar energy and bioeconomy in the United Arab Emirates. Energy Sci. Eng. 7, 1451–1457 (2019).
Boretti, A., Castelletto, S. & Al-Zubaidy, S. Concentrating solar power tower technology: present status and outlook. Nonlinear Eng. 8, 10–31 (2019).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016).
Takata, T. et al. Photocatalytic water splitting with quantum efficiency of almost unity. Nature 581, 411–414 (2020).
Wang, Z. et al. Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles. Nat. Catal. 1, 756–763 (2018).
Wang, Q. et al. Oxysulfide photocatalyst for visible-light-driven overall water splitting. Nat. Mater. 18, 827–832 (2019).
Lyu, H. et al. An Al-doped SrTiO3 photocatalyst maintaining sunlight-driven overall water splitting activity over 1000 h of constant illumination. Chem. Sci. 10, 3196–3201 (2019).
Yamada, T. & Domen, K. Development of sunlight driven water splitting devices towards future artificial photosynthetic industry. ChemEngineering 2, 36 (2018).
Maeda, K. et al. Characterization of Rh-Cr mixed-oxide nanoparticles dispersed on (Ga1-xZnx)(N1-xOx) as a cocatalyst for visible-light-driven overall water splitting. J. Phys. Chem. B 110, 13753–13758 (2006).
Peñas, P. et al. Decoupling gas evolution from water-splitting electrodes. J. Electrochem. Soc. 166, H769–H776 (2019).
Kemppainen, E. et al. Effect of the ambient conditions on the operation of a large-area integrated photovoltaic-electrolyser. Sustain. Energy Fuel 4, 4831–4847 (2020).
Wang, Q. et al. Particulate photocatalyst sheets based on carbon conductor layer for efficient Z-scheme pure-water splitting at ambient pressure. J. Am. Chem. Soc. 139, 1675–1683 (2017).
Sayama, K. & Miseki, Y. Research and development of solar hydrogen production – toward the realization of ingenious photocatalysis-electrolysis hybrid system. Synthesiology (Eng. edn) 7, 79–91 (2014).
Schroeder, V. & Holtappels K. Explosion characteristics of hydrogen-air and hydrogen-oxygen mixtures at elevated pressures. In Proc. International Conference on Hydrogen Safety 120001 (ICHS, 2005).
Acknowledgements
This work was funded by the New Energy and Industrial Technology Development Organization (NEDO), Japan. A part of this work was supported by the Nanotechnology Platform project by the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant number JPMXP09A20UT0004). We are grateful to R. Dobashi and T. Mogi of the University of Tokyo for their advice with regard to gas explosion experiments and safety procedures. We are also grateful to H. Kita of Yamaguchi University and N. Tanihara of Ube Industries, Ltd for providing advice concerning polyimide separation membranes. We thank H. Ito of ARPChem for his support on the construction of the arrayed panel reactor. Finally, we thank all of the technical staff that participated in the fabrication and construction of the complete system.
Author information
Authors and Affiliations
Contributions
K.D. planned and supervised this project. H.N. and T.Y. were responsible for the basic design, on-site design, construction and supervision of the plant. H.N. and T.T. investigated the cocatalyst deposition process, and designed, tested, fabricated and supervised the mass production of the photocatalytic reactors. S.O. and H.T. prepared the SrTiO3:Al photocatalyst on a large scale. M.Y., Y.K., Y.N., R.N. and T.H. prepared the modified photocatalyst. M.N. and N.S. performed electron microscopy. Y.M. examined in situ gas evolution. T.Y. designed and constructed the gas filtration apparatus. T.Y. and H.N. performed the field testing of the entire solar hydrogen production system. H.N., T.Y., S.A, T.W. and K.D. examined the safety issues. T.Y., H.N., T.H. and K.D. discussed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
H.N., T.Y. and K.D. of the University of Tokyo hold patents related to this work (Japanese Unexamined Patent Application publication no. 2021-75,319).
Additional information
Peer review information Nature thanks Frank Osterloh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The photocatalyst panel reactors.
a, The design of a panel reactor unit (625 cm2). b, A diagram showing the structure. c, d, Photographic images of the 3-m2 module consisting of 48 panel reactor units viewed from the front (c) and rear (d).
Extended Data Fig. 2 Photocatalyst sheet microstructures.
a, b, Cross-sectional backscattered electron images acquired by scanning electron microscopy of photocatalyst sheets prepared using a manual sprayer on a flat, clear glass sheet (a) or a program-controlled sprayer on a frosted glass sheet (b). c, d, Cross-sectional images of a photocatalyst deposited by manual spraying on a flat, clear glass sheet, acquired by transmission electron microscopy before (c) and after (d) a field test lasting approximately six months.
Extended Data Fig. 3 The dependence of the water splitting rate of the photocatalyst sheet on the loading amount of the photocatalyst layer.
Small photocatalyst sheets (5 cm × 5 cm) prepared on the flat, clear glass were irradiated with the ultraviolet light emitting diode array. The loading amount of the photocatalyst layer is expressed as a relative value to the case where the modified SrTiO3:Al loading is 0.89 mg cm−2.
Extended Data Fig. 4 Durability of photocatalyst sheets.
a, b, The STH values of small photocatalyst sheets (25 cm2) prepared using manual spraying with flat, clear glass (a) and a program-controlled sprayer with frosted glass (b) during the overall water splitting reaction under continuous irradiation with simulated standard sunlight.
Extended Data Fig. 5 Long-term records of the field test of the 100-m2 photocatalyst panel reactor at Kakioka Research Facility from 22 September to 21 December 2020.
a–f, Solar radiation (a), ultraviolet power (b), oxyhydrogen gas production rate (c), ambient temperature (d), daily STH value (e) and proportion of ultraviolet energy (f). The oxyhydrogen gas was not produced in late December 2020, because of the cold weather freezing the panel reactor units and other equipment. On 2, 6 and 30 October, the gas was directed to the gas separation equipment, without delivery to the gas flowmeter. On 9 and 10 October, the daily STH values were abnormally high. These values are not reliable because of bad weather and the low solar radiation. On 9 and 10 October, the daily STH values were abnormally high. These values are not reliable because of bad weather and the low solar radiation. The data on 18 November and 8 December were not reliable either, owing to malfunction of the soap-film flowmeter.
Extended Data Fig. 6 Structure of the gas separating facility.
a, Schematic of the gas filtration cartridge. b, Schematic of the oxyhydrogen gas separation apparatus. The routes for the incoming oxyhydrogen gas, hydrogen-enriched filtrate gas and oxygen-enriched residual gas are shown in purple, red and black, respectively.
Extended Data Fig. 7 Performance of the gas separation unit connected to the 100-m2 water splitting photocatalyst panel reactor.
The hydrogen concentration in the filtrate gas and the oxygen concentration in the residue gas are intermittently notated along the curves. a, b, The data were acquired on a day with mixed sun and cloud (6 February 2020; a) and a consistently sunny day (1 May 2020; b) at the Kakioka Research Facility. Note that the throttle valve was partially closed at 10:00 am on 1 May 2020 to reduce the gas feed rate.
Extended Data Fig. 8 Ignition test of the panel reactor.
The gas in the area indicated within the red square, with a light receiving area of 70 m2, was ignited by a spark. The spark gap was an assembly of two needle electrodes facing each other within a tube. The distance between the electrodes was adjusted at approximately 0.5 mm. The ignition voltage was 15-kV a.c. and pulsed for approximately 1 s.
Supplementary information
Supplementary Video 1
| Nucleation of moist oxyhydrogen bubbles on a photocatalyst sheet under irradiation from a Xe lamp, played at ten times the original speed.
Supplementary Video 2
| A reactor unit evolving and expelling oxyhydrogen gas bubbles at approximately 13 ml min−1 under a diode array emitting ultraviolet light.
Supplementary Video 3
| Moist oxyhydrogen gas bubbles generated at a rate of 3.7 l min−1 by the 100-m2 photocatalyst panel reactor under natural sunlight on 30 September 2020, played at the original speed.
Supplementary Video 4
| Intentional ignition of the oxyhydrogen gas in polyurethane gas collection and carrying tubes with an inner diameter of 8 mm connected to the water splitting panel reactor with a 70-m2 light receiving area during water splitting under natural sunlight at 10:00 am on 20 August 2020, played in slow motion (1/240 of the original speed). Reactor internal pressure = 101 kPa, temperature = 30 °C. This experiment was conducted twice without damaging the setup.
Supplementary Video 5
| Intentional ignition of a mixed gas of hydrogen and oxygen (H2/O2 = 2) saturated with water vapour, filled in the soft polyvinyl chloride tube with an inner diameter of 10 mm and a length of 100 m with the end bobbed in a water tub, played in the original speed. Internal pressure = 101 kPa, temperature = 20 °C. This experiment was conducted five times without any damage.
Supplementary Video 6
| The same experiment with Supplementary Video 5 but recorded from a different angle and played in slow motion (1/132 of the original speed).
Rights and permissions
About this article
Cite this article
Nishiyama, H., Yamada, T., Nakabayashi, M. et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 598, 304–307 (2021). https://doi.org/10.1038/s41586-021-03907-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03907-3
This article is cited by
-
Solar reforming as an emerging technology for circular chemical industries
Nature Reviews Chemistry (2024)
-
Efficient and stable visible-light-driven Z-scheme overall water splitting using an oxysulfide H2 evolution photocatalyst
Nature Communications (2024)
-
Polyoxometalates-derived nanostructures for electrocatalysis application
Rare Metals (2024)
-
Cobalt-based transition metal boride electrocatalysts for alkaline water oxidation reactions
Ionics (2024)
-
Approaching the commercial threshold of solar water splitting toward hydrogen by III-nitrides nanowires
Frontiers in Energy (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.