Abstract
Coping with threatening situations requires both identifying stimuli that predict danger and selecting adaptive behavioural responses to survive1. The dorsomedial prefrontal cortex (dmPFC) is a critical structure that is involved in the regulation of threat-related behaviour2,3,4. However, it is unclear how threat-predicting stimuli and defensive behaviours are associated within prefrontal networks to successfully drive adaptive responses. Here we used a combination of extracellular recordings, neuronal decoding approaches, pharmacological and optogenetic manipulations to show that, in mice, threat representations and the initiation of avoidance behaviour are dynamically encoded in the overall population activity of dmPFC neurons. Our data indicate that although dmPFC population activity at stimulus onset encodes sustained threat representations driven by the amygdala, it does not predict action outcome. By contrast, transient dmPFC population activity before the initiation of action reliably predicts avoided from non-avoided trials. Accordingly, optogenetic inhibition of prefrontal activity constrained the selection of adaptive defensive responses in a time-dependent manner. These results reveal that the adaptive selection of defensive responses relies on a dynamic process of information linking threats with defensive actions, unfolding within prefrontal networks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors on request.
Code availability
The custom-written codes used to analyse data from this study are available from the corresponding authors on request.
References
Pearce, J. M. & Bouton, M. E. Theories of associative learning in animals. Annu. Rev. Psychol. 52, 111–139 (2001).
Sotres-Bayon, F. & Quirk, G. J. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 20, 231–235 (2010).
Herry, C. & Johansen, J. P. Encoding of fear learning and memory in distributed neuronal circuits. Nat. Neurosci. 17, 1644–1654 (2014).
Klavir, O., Prigge, M., Sarel, A., Paz, R. & Yizhar, O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat. Neurosci. 20, 836–844 (2017).
Laubach, M., Wessberg, J. & Nicolelis, M. A. Cortical ensemble activity increasingly predicts behaviour outcomes during learning of a motor task. Nature 405, 567–571 (2000).
Ährlund-Richter, S. et al. A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat. Neurosci. 22, 657–668 (2019).
Kennerley, S. W., Walton, M. E., Behrens, T. E., Buckley, M. J. & Rushworth, M. F. Optimal decision making and the anterior cingulate cortex. Nat. Neurosci. 9, 940–947 (2006).
Saez, A., Rigotti, M., Ostojic, S., Fusi, S. & Salzman, C. D. Abstract context representations in primate amygdala and prefrontal cortex. Neuron 87, 869–881 (2015).
Salay, L. D., Ishiko, N. & Huberman, A. D. A midline thalamic circuit determines reactions to visual threat. Nature 557, 183–189 (2018).
Hayden, B. Y. & Platt, M. L. Neurons in anterior cingulate cortex multiplex information about reward and action. J. Neurosci. 30, 3339–3346 (2010).
Do-Monte, F. H., Quiñones-Laracuente, K. & Quirk, G. J. A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463 (2015).
Taub, A. H., Perets, R., Kahana, E. & Paz, R. Oscillations synchronize amygdala-to-prefrontal primate circuits during aversive learning. Neuron 97, 291–298 (2018).
Courtin, J. et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505, 92–96 (2014).
Dejean, C. et al. Prefrontal neuronal assemblies temporally control fear behaviour. Nature 535, 420–424 (2016).
Karalis, N. et al. 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nat. Neurosci. 19, 605–612 (2016).
Likhtik, E., Stujenske, J. M., Topiwala, M. A., Harris, A. Z. & Gordon, J. A. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat. Neurosci. 17, 106–113 (2014).
Burgos-Robles, A. et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 20, 824–835 (2017).
Diehl, M. M. et al. Active avoidance requires inhibitory signaling in the rodent prelimbic prefrontal cortex. eLife 7, e34657 (2018).
Bravo-Rivera, C., Roman-Ortiz, C., Brignoni-Perez, E., Sotres-Bayon, F. & Quirk, G. J. Neural structures mediating expression and extinction of platform-mediated avoidance. J. Neurosci. 34, 9736–9742 (2014).
Capuzzo, G. & Floresco, S. B. Prelimbic and infralimbic prefrontal regulation of active and inhibitory avoidance and reward-seeking. J. Neurosci. 40, 4773–4787 (2020).
Yu, B. M. et al. Gaussian-process factor analysis for low-dimensional single-trial analysis of neural population activity. J. Neurophysiol. 102, 614–635 (2009).
Rozeske, R. R. et al. Prefrontal-periaqueductal gray-projecting neurons mediate context fear discrimination. Neuron 97, 898–910.e6 (2018).
Legendre, P. & Legendre, L. Numerical Ecology (Developments in Environmental Modelling) (Elsevier, 1998).
Rigotti, M. et al. The importance of mixed selectivity in complex cognitive tasks. Nature 497, 585–590 (2013).
Choi, J. S., Cain, C. K. & LeDoux, J. E. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn. Mem. 17, 139–147 (2010).
Quirk, G. J., Repa, C. & LeDoux, J. E. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron 15, 1029–1039 (1995).
Duvarci, S. & Pare, D. Amygdala microcircuits controlling learned fear. Neuron 82, 966–980 (2014).
Pape, H. C. & Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–463 (2010).
Goosens, K. A., Hobin, J. A. & Maren, S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: mnemonic code or fear bias? Neuron 40, 1013–1022 (2003).
Senn, V. et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437 (2014).
Sotres-Bayon, F., Sierra-Mercado, D., Pardilla-Delgado, E. & Quirk, G. J. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76, 804–812 (2012).
Poremba, A. & Gabriel, M. Amygdalar lesions block discriminative avoidance learning and cingulothalamic training-induced neuronal plasticity in rabbits. J. Neurosci. 17, 5237–5244 (1997).
Sierra-Mercado, D., Padilla-Coreano, N. & Quirk, G. J. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538 (2011).
Ramirez, F., Moscarello, J. M., LeDoux, J. E. & Sears, R. M. Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J. Neurosci. 35, 3470–3477 (2015).
LeDoux, J. E., Moscarello, J., Sears, R. & Campese, V. The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm. Mol. Psychiatry 22, 24–36 (2017).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Franklin, K. B. J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Academic, 2001).
Meyers, E. M., Qi, X. L. & Constantinidis, C. Incorporation of new information into prefrontal cortical activity after learning working memory tasks. Proc. Natl Acad. Sci. USA 109, 4651–4656 (2012).
Acknowledgements
We thank D. Salzman, A. Luthi, K. Benchenane, S. Bagur and R. Rozeske for discussions and reading of the manuscript; A. Sant Ana and S. Khoder for preliminary experiments; T. Stefanelli for electrode building assistance; K. Deisseroth and E. Boyden for sharing material; and S. Laumond, J. Tessaire and the technical staff of the housing and experimental animal facility of the Neurocentre Magendie. Microscopy was performed in the Bordeaux Imaging Center of the CNRS-INSERM and Bordeaux University, a member of France BioImaging. This work was supported by grants from the French National Research Agency (ANR-DOPAFEAR, ANR-PRELONGIN, ANR-10-EQPX-08 OPTOPATH), the Conseil Regional d’Aquitaine, and the Fondation pour la Recherche Médicale (FRM-Equipes FRM 2017).
Author information
Authors and Affiliations
Contributions
D.J., N.W. and K.S. performed electrophysiological recordings. D.J. and N.W. performed optogenetics and pharmacological experiments. D.J., N.W. and C.F. performed histology. D.J., N.W., C.F. and D.R. performed behavioural experiments on nonimplanted mice. M.M.F. performed preliminary viral injections. J.C. and F.C. provided tone fear conditioning data. D.J., S.V. and C.H. designed the experiments. D.J. and P.E.J. designed analyses. D.J. wrote software codes. D.J. and C.H. analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Andrew Huberman, Joshua Johansen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
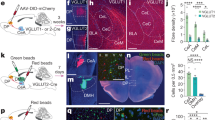
Extended Data Fig. 1 Discriminative two-way active avoidance behaviour.
a, The number of trials until first CS+ NAV trial significantly increases until day 3 (***P < 0.001 for CS+, two-way repeated measures (RM) ANOVA) (left). Avoidance latencies (centre) or non-avoidance (right) latencies were unaltered after day 3 (*P < 0.05 for CS+, two-way RM ANOVA; n = 34 mice). b, Distribution of avoidance latencies across days for CS− (left) and CS+ (right) trials. Vertical dashed lines show distribution means per day. c, Freezing was rapidly expressed after training started (left). Mean freezing across days significantly differed between CS− and CS+ on day 3 and 4 (right) (*P = 0.040 **P = 0.0075, two-way RM ANOVA; n = 34 mice). d, Detection sensitivity (d′, top) and response bias (d′ criterion, bottom) showing that high discrimination between CS− and CS+ achieved at day 2; response bias is only observable at day 1 (d′ criterion >0 bias towards NAV). e, Extinction training (nonimplanted mice) for short (4 days) (top) (P < 0.001 for 1st block of day 5, two-way RM ANOVA; n = 8 mice) and long training (11 days) (bottom) (P < 0.001 for 1st–3rd and 7th block, two-way RM ANOVA; n = 8 mice). f, Contingency degradation, US (1-s maximum) delivered during inter-trial intervals (*P < 0.05 **P < 0.01 ***P < 0.001, two-way RM ANOVA; n = 8 mice). g, Permanence map during the entire sessions across days for an example mouse, showing the time spent in the different regions of the shuttlebox (colour axis in log scale). h, Probability distributions along the maze across days. i, Trajectories for different trial types and response outcomes for the example mouse shown in g at day 4. White circles indicate the location of the mouse at CS onset, grey circles indicate the location after 7 s (AV (left) and NAV (right); CS− (top), CS+ (bottom)). j, Mouse spatial location at CS onset for AV (top) and NAV (bottom) trials does not predict avoidance behaviour (n = 68 sessions; P = 0.548, two-sampled Kolmogorov–Smirnov test of both cumulative distributions for x-axis position; day 3 and day 4 sessions). Learning curves show five-trial blocks. Error bars showing s.e.m. Error shaded areas are 95% confidence interval (CI).
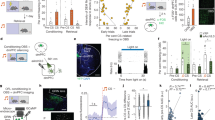
Extended Data Fig. 2 dmPFC pharmacological inactivation and speed-related analyses.
a, Bilateral cannula tip position (n = 12 mice). b, Protocol for pharmacological inactivation experiments. c, Avoidance probability for CS− (left) and CS+ (right) during training for muscimol (n = 7 mice) and vehicle (n = 5 mice) groups. d, Number of infrared beam breaks 2 min before session starts during pre-infusion, infusion and post-infusion sessions for muscimol and vehicle groups, showing that muscimol infusion did not affect basal levels of locomotion (P > 0.9999, RM mixed-effects model). e, CS+ active avoidance was impaired upon dmPFC inactivation (**P = 0.0285, two-way RM ANOVA; outliers indicated as dots) whereas CS− avoidance probability did not change (P = 0.0761, two-way RM ANOVA). f, Mean freezing probability dynamics during CS−, CS+ AV and CS+ NAV trials. g, Percentage freezing during sound presentation for CS−, CS+ AV and CS+ NAV trials on day 3 and 4 (violin plot median and 25th–75th percentiles; ***P < 0.001 one-way RM ANOVA; n = 68 sessions; n = 34 mice). h, CS-onset-triggered median speed for CS−, CS+ AV and CS+ NAV trials during the habituation session (left) and during training day 3 and 4 (right). For day-3–4 sessions, trials were only included in the analyses if the AV shuttle time was a minimum of 2.5 s. i, CS-onset-triggered median speed for CS+ AV trials grouped by different AV latencies. In all cases a drop in speed at CS onset was observed, preceding the sharp increase in speed associated with avoidance responses. j, Schematics of AV-run alignment definition. Instantaneous acceleration is computed for CS+ AV individual trials and AV-run initiation is defined by the first event in which acceleration surpasses a threshold (50 cm per s−2; minimum AV-run time of 2.5 s were included in the analyses). The same alignment was used for CS+ NAV (and CS−) trials, by randomly assigning AV-run times from CS+ AV trials. k, Individual AV-run-aligned speed traces from CS+ AV and CS+ NAV trials (top). AV-run and avoidance (hurdle crossing) time distributions for CS+ AV trials (bottom-left). Time difference between AV-run initiation and hurdle crossing distribution (bottom right). l, Median speed at CS-onset-aligned (left) and AV-run-aligned (right) conditions. AV-run alignment for both CS+ NAV and CS− defined by sampling CS+ AV-run times (Methods). m, Learning curves during training in which the tones used for CS− and CS+ were switched (left; ***P < 0.0001 for 7th block forward, two-way RM ANOVA; n = 9 mice). CS-onset-triggered median speed for different trials type at day 3 and day 4 for switched tones (right). Error bars are s.e.m. Error shaded areas are 95% CI.
Extended Data Fig. 3 In vivo single-unit recordings in the dmPFC.
a, Representative electrolytic lesion (left) and electrode tip locations for recorded mice (centre, n = 34 mice). Example of single unit recorded in the dmPFC showing the average waveforms, isolation in the principal component (PC) space and autocorrelogram (right). b, Area under the curve (AUC) and trough-to-peak distance were used to separate units into putative excitatory (exc.) and inhibitory (inh.), using k-means clustering (k = 2). Individual classified waveforms and percentages shown. c, Violin plots displaying average firing rates across the entire session for individual units during habituation (day 0, 872 units) and at high levels of avoidance (day 4, 711 units) do not show significant differences (P = 0.3519, unpaired t-test). d, Z-scored neuronal activity for CS+ (left) and CS− (right) trials ordered by the magnitude of CS− responses (same data as in Fig. 1d; day 3 and 4 data pooled, 68 sessions, n = 1,261 units). e, Heat maps displaying z-scores of the population of recorded units for different trial types (n = 34 sessions, 643 units displayed; units with undefined z-scores (n = 22 units) were removed). f, Percentage of total units (n = 643 units) significantly responding to CS+ or CS− during the first 7 s after CS onset. For each category, the percentage of units showing excitatory and inhibitory responses (these are not mutually exclusive categories, as a unit can display both excited and inhibited responses). h, Average spectrograms of dmPFC local field potentials (LFP) during spontaneous freezing and nonfreezing periods (n = 14 mice). i, Power spectra during nonfreezing and freezing periods. Inset displays ‘4 Hz’ mean signal-to-noise (P = 0.0015, Wilcoxon test; error bar are s.e.m.). j, During the active avoidance task, average spectrograms during different trial types and responses. k, Corresponding power spectra for different trial types and responses together with pre-CS periods. l, Average spectrogram differences highlighting the changes for different trial types. m, Power spectra for different trial types. Shaded error areas are 95% CI.
Extended Data Fig. 4 Neuronal activity at AV-run alignment.
a, Examples of different unit response patterns observed at CS-onset- and AV-run-aligned activity (for each case: raster on top, average firing rate and speed on bottom). Each column shows examples of different observed patterns. b, Z-scored activity for different unit responses during CS+ AV (left), CS+ NAV (centre) and CS− (right) trials, ordered by the magnitude of CS+ AV responses (here we considered sessions with at least 6 trials of each type; n = 33 sessions, 611 units displayed; units with undefined z-scores (n = 23 units) were removed). c, Average firing rates across units for different trial type. d, Average z-score across units for different trial types. e, Mean prediction error of GPFA as a function of the number of latent dimensions considered (following a previous publication21) for CS-onset- (top) and AV-run- (bottom) aligned conditions (error bard are s.e.m.). Shaded error areas are 95% CI.
Extended Data Fig. 5 CS decoding from dmPFC neuronal populations.
a, Tone fear conditioning protocol. b, During habituation (day 0), mice (n = 38 mice) exhibited low mean freezing during CS− and CS+. Following fear conditioning (FC) (day 1, mice n = 38 mice), CS+ (CS+ 1–12 grouped in blocks of 4) induced high mean freezing in comparison to CS− (P < 0.0001, one-way RM ANOVA; n = 38 mice). c, Freezing probability as a function of time during 4 CS− and 4 (first) CS+. d, Decoding accuracy of CS+ (top) and CS− (bottom) from baseline population activity for tone-fear-conditioned mice on habituation (day 0, n = 553 units; n = 38 sessions). e, As in d, but post-FC (day 1, n = 582 units; n = 38 sessions). f, Mean avoidance probability during extinction learning (n = 14 mice). Although CS+ avoidance behaviour (top) was extinguished after the first extinction session (**P < 0.0110 for 1st and 3rd block, two-way RM ANOVA), CS+ from baseline decoding accuracy (bottom) was reduced only on the subsequent extinction session. g, Extinction training avoidance and shuttling mean probability during 12 s (maximal CS duration, in dark colours) or 7 s (as in f top, in light colours) after CS onset. After the first half of the first extinction session, CS+ shuttling decreases and CS− increases reaching similar values to those observed during the habituation session (n = 14 mice). h, Average CS+-evoked freezing during the first (day 6) (top) and second (day 7) (bottom) extinction session, for the first (light red) and last (dark red) half of the session. i, Mean avoidance probability during reversal learning (n = 11 mice). Previous CS+ tone became neutral (reversed CS− (rCS−)) and the previous CS− tone was associated with the US (rCS+). Avoidance behaviour was progressively reversed during training (top) (**P < 0.01 *P < 0.05 difference between rCS− and rCS+, two-way RM ANOVA) and decoding accuracies changed accordingly (bottom). j, Assessing safety signal properties on CS−. Two groups of mice (n = 8 mice each) were trained in normal protocol until day 4 (top). Retardation test was performed during the 3 subsequent days in one of the groups (group A) (bottom left) by pairing CS− with the US, the transfer of active avoidance behaviour matched the initial AV probability to the CS+ only at day 7. ns, P = 0.0691 for CS− versus CS+ at day 3, two-way RM ANOVA. Additionally, to test the effect of a new association in the learning rate in another group of mice (group B) we paired a novel tone with the US (bottom right), which led to a rapid transfer of active avoidance behaviour (ns, P = 0.2133 for CSx versus CS+ day 3, two-way RM ANOVA). Our data suggest that CS− acquired safety properties (‘CS− with no US’ association) during the initial training. Significant decoding accuracy periods over shuffle accuracies were represented by thick lines (P < 0.05, permutation test; Methods). Error bars are s.e.m. Accuracy data are mean ± 1 s.d.
Extended Data Fig. 6 Confined task and threat information decoding at CS onset.
a, After training, mice were confined to one of the compartments where unreinforced conditioned stimuli were presented (confined task; n = 21 mice). Median speed dynamics in the confined task showed decreased speed selective to CS+ trials. b, Mean freezing probability dynamics during CS− and CS+ trials in the confined task. c, Freezing to CS+ progressively increases during the first 3 CS+ trials (5th trial) and reaches stable levels from trial 8 to 12 (***P < 0.001, one-way RM ANOVA; n = 21 mice). d, Average spectrograms for different trial types during the confined task (n = 11 mice). e, Spectrogram difference between CS+ and CS−. f, Power spectra for CS+ and CS− in the confined task. Inset displays mean ‘4 Hz’ signal-to-noise (P = 0.02, Wilcoxon test). g, Freezing probabilities when mice exhibit freezing (CS+ FzOns) or nonfreezing (CS+ NFzOns) at CS onset, light red area indicates the period in which mice showed pure freezing or nonfreezing bouts, respectively (left) (n = 13 sessions) (Methods). Decoding CS+ FzOns and CS+ NFzOns by dmPFC activity (right). h, Decoding spontaneous freezing (outside CS) from nonfreezing episodes (minimum episode durations of 4 s) (left). Decoding spontaneous freezing (outside CS) and CS+ (right). i, Decoding accuracy of CS+ (top) and CS− (bottom) from baseline population activity during the confined task. j, Trained decoders to classify CS+ trials from baseline in the active avoidance task (CS+AA) displayed high accuracy when decoding CS+ in the confined task (CS+CNF). k, Trained decoders to classify CS+ trials from baseline in the confined task (CS+CNF) displayed high accuracy when decoding CS+ in the active avoidance task (CS+AA). To compare the information across panels, decoding was performed by randomly sampling 250 units in each case. Significant decoding accuracy periods over shuffle accuracies are represented by thick lines (P < 0.05, permutation test) (Methods). Error bars are s.e.m. Error shaded areas are 95% CI. Accuracy data are mean ± 1 s.d.
Extended Data Fig. 7 Influence of unit amount and population versus single-mouse CS decoding.
a, Decoding accuracy of CS+ from baseline activity for individual units from day 3 and day 4. Average accuracy across single units displayed in light grey. b, Cumulative distribution of mean single-unit decoding accuracies during 4 s after CS onset. c, Effect of the number of units in the amount of decoding accuracy considering single-unit, multi-unit (as the summation of single-units) and the multidimensional population vector. We refer to the population vector as the pseudopopulation vector that is composed of the firing rate from individual units (pooled from different sessions). d, Mean decoding accuracy during 4 s after CS onset across days on which the population vector was formed by pooling units from different mice (250 units randomly sampled for each of the bootstrap repetition; bootstrap 200 repetitions, shuffled label 1,000 repetitions, fivefold cross-validation; *P < 0.05, permutation test; Error bars represent 1 s.d.). e, Mean decoding accuracies in which population vector is formed by units from individual mice (n = 14 mice; for each mouse, bootstrap 200 repetitions, shuffled label 1,000 repetitions, fivefold cross-validation; real and shuffle accuracies were pooled across mice to assess statistical significance, * P < 0.05, permutation test; error bars represent 1 s.e.m.). Dashed horizontal lines indicate accuracy chance level.
Extended Data Fig. 8 dmPFC threat representations depend on amygdala processing.
a, Cannula tip placement for behaviour-only experiments targeting the BLA (n = 11 mice). b, Mice received training until day 3. On day 4, 1 h before the session started, mice were infused with vehicle (n = 5 mice) or muscimol (n = 6 mice). c, Avoidance probability for CS− (top) and CS+ (bottom) during habituation and training for both muscimol and vehicle groups (ns, P > 0.9999, RM mixed-effects model). d, Number of infrared beam breaks 2 min before session starts during pre-infusion (Pre), infusion (Infus.) and post-infusion (Post) sessions, showing that muscimol did not affect basal levels of locomotion on infusion session (but changed the post-infusion session; **P = 0.0083, RM mixed-effects model). e, CS+ active avoidance was impaired upon BLA muscimol inactivation (***P < 0.0001, RM mixed-effects model; outliers indicated as dots) whereas CS− avoidance probability did not change (ns, P = 0.9424, RM mixed-effects model) f, Cannula tip location targeting the BLA and electrode implantation site in the dmPFC (n = 11 mice). g, Muscimol infusion (Mus) in the BLA impairs active avoidance behaviour to CS+ (***P < 0.0001, **P < 0.001, one-way RM ANOVA) but not CS− (ns, P = 0.2929, one-way RM ANOVA). h, CS decoding accuracies from dmPFC activity, obtained during the pre-session (day 3). i, CS decoding accuracy from dmPFC activity during BLA muscimol inactivation. Despite the fact that decoding accuracies in between pips are markedly reduced, we still decode the auditory sensory stimuli at pip times. j, CS decoding accuracy from dmPFC activity the post-session after BLA inactivation returns to previous levels of decoding accuracy. k, Neuronal population trajectories for CS+ and CS− presentations during Pre (day 3) (left) and Mus (day 4) (centre) sessions projected onto the first 3 latent dimensions (30 trials for each trial type shown). Circles indicate time zero. Arrows schematize trajectory flow. Mean ± s.d. of normalized distance (d) between CS+ and CS− for Pre (light blue) and Mus (dark blue) conditions. Thick lines indicate significantly increased normalized distance (P < 0.05, permutation test). Horizontal coloured lines indicate periods in which mean accuracy is significantly different from those observed from the shuffle accuracy distribution (P < 0.05, permutation test) (Methods). To compare the information across panels, decoding was performed by randomly sampling 250 units for each bootstrap repetition. Error bars are s.e.m. Accuracy data are mean ± 1 s.d.
Extended Data Fig. 9 Avoidance action coding by dmPFC populations.
a, To preclude the possibility that a single session inordinately contributed to the observed surge in AV versus NAV decoding accuracy before action initiation, a jackknife procedure was used by repeatedly removing an individual session (n = 33 sessions in total) to compute the decoding accuracy at AV-run-aligned condition. b, Distributions of the number of CS+ NAV and CS+ AV trials for different sessions (Methods). c, Number of sessions and units as a function of the minimum number of trials available for both the CS+ NAV and CS+ AV trial types. d, Decoding CS+ NAV versus CS+ AV using a different number of minimum trials for session inclusion criteria. e, Mean (top) and maximum (bottom) accuracy from −1 to 0 s to AV-run for the different number of trials for session inclusion criteria (error bars display s.e.). f, AV-run-triggered snout, centre-of-mass and tail average speed for CS+ AV (left) and CS+ NAV (right) trials. g, CS+ AV and CS+ NAV speed discrimination index d′. h, dmPFC neural activity and speed-based decoding accuracy for different body parts (as in Fig. 3). i, Transition from freezing to nonfreezing is not preceded by a long time-scale surge in decoding accuracy in dmPFC populations. Spontaneous freezing episodes (longer than 4 s) were both aligned to freezing-to-nonfreezing transition times (Fz > NFz) or 2 s before this freezing-to-freezing transition (Fz > Fz) (left) (Methods). Median speed for Fz > NFz and Fz > Fz trials (centre top) and discrimination index d′ (centre bottom). dmPFC neural activity based Fz > NFz vs Fz > Fz decoding accuracy (dark grey) is compared with AV versus NAV decoding accuracy (light grey) in AV-run-aligned condition (right). j, Heat map displaying z-score of CS+ evoked activity for single units ordered by the magnitude of the response (left). For each unit and in the same order, the corresponding mean feature weight (2 s after CS onset) in the linear classifiers for the CS+/baseline activity. k, Mean feature weight for CS+/baseline versus CS+ AV/NAV before action initiation for individual units were not correlated (r = 0.0069, P = 0.09, Pearson correlation). l, Decoder accuracies from dmPFC activity (top) and multidimensional (snout, centre and tail) speed signals (bottom), when activity is aligned at CS onset (left) or AV-run (right). m, Comparison between dmPFC activity-based and multidimensional speed-based decoding accuracies across the different transition to movement: spontaneous freezing to nonfreezing transitions (left), spontaneous snout-movement without running (run detection based on snout speed, but no detection on centre speed) (centre) and spontaneous run (right). Decoding based on dmPFC activity was computed using the full number of units (in blue: 1,276 units (left), 1,089 units (centre) and 1,475 units (right)) or subsampling the number of units used for decoding CS+ AV from CS+ NAV activity (black: 634 units). Horizontal coloured lines indicate periods in which mean accuracy is significantly different from those observed from the shuffle accuracy distribution (P < 0.05, permutation test; Methods). Accuracy data are mean ± 1 s.d.
Extended Data Fig. 10 Photoinactivation of dmPFC and BLA-to-dmPFC projections in the active avoidance task.
a, Optic fibre tip location for dmPFC inactivation experiments. Shaded areas are showing virus spreading. b, Median speed for different trial types of the ArchT groups for laser off and on conditions. c, Body length (measured as the distance between snout and tail) during the time course of CS− (top) and CS+ NAV (bottom) trials for off and on conditions displaying overall stable values. d, Snout median speed during the time course of CS− (top) and CS+ NAV (bottom) trials for off and on conditions. e, Injection of retro-ArchT and optrode implantation in the dmPFC. Light delivery induced changes in neuronal activity in the dmPFC (13 units recorded, n = 1 mouse). f, Micrograph example of dmPFC projecting cells in the BLA labelled by the retro-ArchT stratergy. Scale bars, 100 μm. g, Comparison of light diffusion in 0.6% agar of standard 200 μm core 0.39 NA with 400 μm core 0.66 NA fibres (scale bar, 1 mm; 10 mW measured at the tip of each optic fibre). We used the 400 μm core 0.66 NA optic fibres to maximize the illuminated area. h, Location of fibre tips in the amygdala (retro-ArchT n = 11 mice, GFP n = 6 mice). i, Avoidance probability at day 3 compared to day 4, in which all trials were stimulated, did not significantly differ (ns, P = 0.9771 for CS+ and P = 0.6671 for CS−, RM mixed-effects model). j, CS decoding accuracies on day 3 and day 4, both using 85 units (n = 5 mice). Thick horizontal lines indicate significant decoding accuracy values compared to the shuffle condition (P < 0.05, permutation test) (Methods). k, On day 5, we performed a stimulation protocol of 10 first trials with laser off followed by 10 trials of laser on. Decoding CS+ in laser on from laser off conditions (ns, P > 0.065, permutation test). l, Schematics of an alternative model for threat and action processing in the dmPFC. The dmPFC represents threat information inputs coming from upstream structures in a sustained manner. A downstream structure integrates that information from the dmPFC, implementing a decision mechanism such as an integrator-to-bound mechanism (top). Once the decision variable reaches a certain threshold (avoidance decision), broadcast information about the impending movement is reflected in the dmPFC (bottom). Speed error bars are 95% CI. Accuracy data are mean ± 1 s.d.
Supplementary information
Supplementary Discussion
This file contains Supplementary text and supplementary references.
Supplementary Table 1
Summary of statistical results associated with the Figures and Extended Data Figures.
41586_2021_3726_MOESM5_ESM.mp4
Supplementary Video 2 Supplementary Video 2. dmPFC population activity at AV-run. Temporal evolution of neural trajectories assessed by GPFA showed for 30 CS+AV, CS+NAV and CS- trials for AV-run aligned activity. Time indicated on top (red). Related to Figure 1i.
41586_2021_3726_MOESM6_ESM.mp4
Supplementary Video 1 Supplementary Video 1. dmPFC population activity at CS onset. Temporal evolution of neural trajectories assessed by GPFA showed for 30 CS+AV, CS+NAV and CS- trials for CS onset aligned activity. Time indicated on top (red). Related to Figure 1i.
Rights and permissions
About this article
Cite this article
Jercog, D., Winke, N., Sung, K. et al. Dynamical prefrontal population coding during defensive behaviours. Nature 595, 690–694 (2021). https://doi.org/10.1038/s41586-021-03726-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03726-6
This article is cited by
-
The dynamic state of a prefrontal–hypothalamic–midbrain circuit commands behavioral transitions
Nature Neuroscience (2024)
-
A distinct cortical code for socially learned threat
Nature (2024)
-
Prefrontal circuits encode both general danger and specific threat representations
Nature Neuroscience (2023)
-
Early selection of task-relevant features through population gating
Nature Communications (2023)
-
Activity-dependent organization of prefrontal hub-networks for associative learning and signal transformation
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.