Abstract
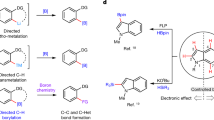
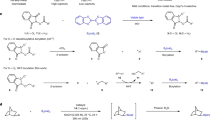
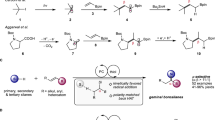
Boron functional groups are often introduced in place of aromatic carbon–hydrogen bonds to expedite small-molecule diversification through coupling of molecular fragments1,2,3. Current approaches based on transition-metal-catalysed activation of carbon–hydrogen bonds are effective for the borylation of many (hetero)aromatic derivatives4,5 but show narrow applicability to azines (nitrogen-containing aromatic heterocycles), which are key components of many pharmaceutical and agrochemical products6. Here we report an azine borylation strategy using stable and inexpensive amine-borane7 reagents. Photocatalysis converts these low-molecular-weight materials into highly reactive boryl radicals8 that undergo efficient addition to azine building blocks. This reactivity provides a mechanistically alternative tactic for sp2 carbon–boron bond assembly, where the elementary steps of transition-metal-mediated carbon–hydrogen bond activation and reductive elimination from azine-organometallic intermediates are replaced by a direct, Minisci9-style, radical addition. The strongly nucleophilic character of the amine-boryl radicals enables predictable and site-selective carbon–boron bond formation by targeting the azine’s most activated position, including the challenging sites adjacent to the basic nitrogen atom. This approach enables access to aromatic sites that elude current strategies based on carbon–hydrogen bond activation, and has led to borylated materials that would otherwise be difficult to prepare. We have applied this process to the introduction of amine-borane functionalities to complex and industrially relevant products. The diversification of the borylated azine products by mainstream cross-coupling technologies establishes aromatic amino-boranes as a powerful class of building blocks for chemical synthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Blakemore, D. in Synthetic Methods in Drug Discovery Vol. 1 (eds Blakemore, D. C., Doyle, P. M. & Fobian, Y. M.) 1–69 (Royal Society of Chemistry, 2016).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C–B bonds. Chem. Rev. 110, 890–931 (2010).
Larsen, M. A. & Hartwig, J. F. Iridium-catalyzed C–H borylation of heteroarenes: scope, regioselectivity, application to late-stage functionalization, and mechanism. J. Am. Chem. Soc. 136, 4287–4299 (2014).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Staubitz, A., Robertson, A. P. M., Sloan, M. E. & Manners, I. Amine− and phosphine−borane adducts: new interest in old molecules. Chem. Rev. 110, 4023–4078 (2010).
Baban, J. A., Marti, V. P. J. & Roberts, B. P. Ligated boryl radicals. Part 2. Electron spin resonance studies of trialkylamin–boryl radicals. J. Chem. Soc. Perkin Trans. 2 1723–1733 (1985).
Duncton, M. A. J. Minisci reactions: versatile CH-functionalizations for medicinal chemists. MedChemComm 2, 1135–1161 (2011).
Hall, D. G. in Boronic Acids (ed. Hall, D. G.) 1–99 (Wiley, 2005).
West, M. J., Fyfe, J. W. B., Vantourout, J. C. & Watson, A. J. B. Mechanistic development and recent applications of the Chan–Lam amination. Chem. Rev. 119, 12491–12523 (2019).
Furuya, T., Kaiser, H. M. & Ritter, T. Palladium-mediated fluorination of arylboronic acids. Angew. Chem. Int. Ed. 47, 5993–5996 (2008).
Bonet, A., Odachowski, M., Leonori, D., Essafi, S. & Aggarwal, V. K. Enantiospecific sp2–sp3 coupling of secondary and tertiary boronic esters. Nat. Chem. 6, 584–589 (2014).
Ishiyama, T., Murata, M. & Miyaura, N. Palladium(0)-catalyzed cross-coupling reaction of alkoxydiboron with haloarenes: a direct procedure for arylboronic esters. J. Org. Chem. 60, 7508–7510 (1995).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Sadler, S. A. et al. Iridium-catalyzed C–H borylation of pyridines. Org. Biomol. Chem. 12, 7318–7327 (2014).
Brown, D. G., Gagnon, M. M. & Boström, J. Understanding our love affair with p-chlorophenyl: present day implications from historical biases of reagent selection. J. Med. Chem. 58, 2390–2405 (2015).
Cook, X. A. F., de Gombert, A., McKnight, J., Pantaine, L. R. E. & Willis, M. C. The 2-pyridyl problem: challenging nucleophiles in cross-coupling arylations. Angew. Chem. Int. Ed. 60, 11068–11091 (2020).
Li, J. et al. Synthesis of many different types of organic small molecules using one automated process. Science 347, 1221–1226 (2015).
Dick, G. R., Woerly, E. M. & Burke, M. D. A general solution for the 2-pyridyl problem. Angew. Chem. Int. Ed. 51, 2667–2672 (2012).
Proctor, R. S. J. & Phipps, R. J. Recent advances in Minisci-type reactions. Angew. Chem. Int. Ed. 58, 13666–13699 (2019).
Taniguchi, T. Boryl radical addition to multiple bonds in organic synthesis. Eur. J. Org. Chem. 2019, 6308–6319 (2019).
Curran, D. P. et al. Synthesis and reactions of N-heterocyclic carbene boranes. Angew. Chem. Int. Ed. 50, 10294–10317 (2011).
Giles, J. R. M. & Roberts, B. P. An electron spin resonance study of the generation and reactions of borane radical anions in solution. J. Chem. Soc. Perkin Trans. 2 743–755 (1983).
Mills, H. A., Martin, J. L., Rheingold, A. L. & Spokoyny, A. M. Oxidative generation of boron-centered radicals in carboranes. J. Am. Chem. Soc. 142, 4586–4591 (2020).
Yang, L., Uemura, N. & Nakao, Y. Meta-selective C–H borylation of benzamides and pyridines by an iridium–Lewis acid bifunctional catalyst. J. Am. Chem. Soc. 141, 7972–7979 (2019).
Mkhalid, I. A. I. et al. Ir-catalyzed borylation of C–H bonds in N-containing heterocycles: regioselectivity in the synthesis of heteroaryl boronate esters. Angew. Chem. Int. Ed. 45, 489–491 (2006).
Preshlock, S. M. et al. A traceless directing group for C–H borylation. Angew. Chem. Int. Ed. 52, 12915–12919 (2013).
Chotana, G. A., Rak, M. A. & Smith, M. R. Sterically directed functionalization of aromatic C−H bonds: selective borylation ortho to cyano groups in arenes and heterocycles. J. Am. Chem. Soc. 127, 10539–10544 (2005).
Minisci, F. et al. Polar effects in free-radical reactions. Selectivity and reversibility in the homolytic benzylation of protonated heteroaromatic bases. J. Org. Chem. 51, 476–479 (1986).
Ueng, S.-H. et al. Complexes of borane and N-heterocyclic carbenes: a new class of radical hydrogen atom donor. J. Am. Chem. Soc. 130, 10082–10083 (2008).
Dai, W., Geib, S. J. & Curran, D. P. 1,4-Hydroboration reactions of electron-poor aromatic rings by N-heterocyclic carbene boranes. J. Am. Chem. Soc. 142, 6261–6267 (2020).
Hioe, J., Karton, A., Martin, J. M. L. & Zipse, H. Borane–Lewis base complexes as homolytic hydrogen atom donors. Chem. Eur. J. 16, 6861–6865 (2010).
Tehfe, M.-A. et al. N-heterocyclic carbene-borane radicals as efficient initiating species of photopolymerization reactions under air. Polym. Chem. 2, 625–631 (2011).
Roberts, P. B. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 28, 25–35 (1999).
Wu, C., Hou, X., Zheng, Y., Li, P. & Lu, D. Electrophilicity and nucleophilicity of boryl radicals. J. Org. Chem. 82, 2898–2905 (2017).
Sheeller, B. & Ingold, K. U. Absolute rate constants for some reactions of the triethylamine-boryl radical and the borane radical anion. J. Chem. Soc. Perkin Trans. 2 480–486 (2001).
Lalevée, J., Tehfe, M. A., Allonas, X. & Fouassier, J. P. Boryl radicals as a new photoinitiating species: a way to reduce the oxygen inhibition. Macromolecules 41, 9057–9062 (2008).
Jin, J. & MacMillan, D. W. C. Direct α-arylation of ethers through the combination of photoredox-mediated C–H functionalization and the Minisci reaction. Angew. Chem. Int. Ed. 54, 1565–1569 (2015).
Bietti, M. Activation and deactivation strategies promoted by medium effects for selective aliphatic C−H bond functionalization. Angew. Chem. Int. Ed. 57, 16618–16637 (2018).
De Vleeschouwer, F., Speybroeck, V. V., Waroquier, M., Geerlings, P. & Proft, F. D. Electrophilicity and nucleophilicity index for radicals. Org. Lett. 9, 2721–2724 (2007).
Baban, J. A. & Roberts, B. P. An electron spin resonance study of phosphine-boryl radicals; their structures and reactions with alkyl halides. J. Chem. Soc. Perkin Trans. 2 1717–1722 (1984).
Davis, H. J., Mihai, M. T. & Phipps, R. J. Ion pair-directed regiocontrol in transition-metal catalysis: a meta-selective C–H borylation of aromatic quaternary ammonium salts. J. Am. Chem. Soc. 138, 12759–12762 (2016).
Cox, P. A. et al. Base-catalyzed aryl-B(OH)2 protodeboronation revisited: from concerted proton transfer to liberation of a transient aryl anion. J. Am. Chem. Soc. 139, 13156–13165 (2017).
Molloy, J. J. et al. Chemoselective oxidation of aryl organoboron systems enabled by boronic acid-selective phase transfer. Chem. Sci. 8, 1551–1559 (2017).
Kuninobu, Y., Nagase, M. & Kanai, M. Benzylic C(sp3)–H perfluoroalkylation of six-membered heteroaromatic compounds. Angew. Chem. Int. Ed 54, 10263–10266 (2015).
Garattini, E. & Terao, M. Aldehyde oxidase and its importance in novel drug discovery: present and future challenges. Expert Opin. Drug Discov. 8, 641–654 (2013).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Chu, Q. et al. Ionic and organometallic reductions with N-heterocyclic carbene boranes. Chem. Eur. J. 15, 12937–12940 (2009).
Acknowledgements
D.L. thanks the EPSRC for a fellowship (EP/P004997/1) and the European Research Council for a research grant (758427); J.H.K. thanks Marie Curie Actions for a fellowship (842422). The Chemical Capabilities team of Janssen Research and Development (Toledo) is acknowledged for technical support.
Author information
Authors and Affiliations
Contributions
J.H.K. and D.L. designed the project; J.H.K., T.C., M.S. and J.L. performed the experiments; N.S.S. performed the computational studies; and all the authors analysed the results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Information.
Rights and permissions
About this article
Cite this article
Kim, J.H., Constantin, T., Simonetti, M. et al. A radical approach for the selective C–H borylation of azines. Nature 595, 677–683 (2021). https://doi.org/10.1038/s41586-021-03637-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03637-6
This article is cited by
-
Electrochemical reactor dictates site selectivity in N-heteroarene carboxylations
Nature (2023)
-
Tunably strained metallacycles enable modular differentiation of aza-arene C–H bonds
Nature Communications (2023)
-
Atroposelective hydroarylation of biaryl phosphines directed by phosphorus centres
Nature Communications (2023)
-
Photoinduced radical–ionic dihalogen transfer to carbon–carbon multiple bonds using oxime-based surrogates
Nature Synthesis (2023)
-
Designing a photo-assisted Co-C3N4 cathode for high performance Li-O2 batteries
Nano Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.