Abstract
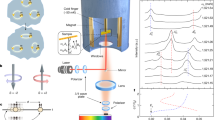
Our understanding of the dielectric response of interfacial water, which underlies the solvation properties and reaction rates at aqueous interfaces, relies on the linear response approximation: an external electric field induces a linearly proportional polarization. This implies antisymmetry with respect to the sign of the field. Atomistic simulations have suggested, however, that the polarization of interfacial water may deviate considerably from the linear response. Here we present an experimental study addressing this issue. We measured vibrational sum-frequency generation spectra of heavy water (D2O) near a monolayer graphene electrode, to study its response to an external electric field under controlled electrochemical conditions. The spectra of the OD stretch show a pronounced asymmetry for positive versus negative electrode charge. At negative charge below 5 × 1012 electrons per square centimetre, a peak of the non-hydrogen-bonded OD groups pointing towards the graphene surface is observed at a frequency of 2,700 per centimetre. At neutral or positive electrode potentials, this ‘free-OD’ peak disappears abruptly, and the spectra display broad peaks of hydrogen-bonded OD species (at 2,300–2,650 per centimetre). Miller’s rule1 connects the vibrational sum-frequency generation response to the dielectric constant. The observed deviation from the linear response for electric fields of about ±3 × 108 volts per metre calls into question the validity of treating interfacial water as a simple dielectric medium.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Miller, R. C. Optical second harmonic generation in piezoelectric crystals. Appl. Phys. Lett. 5, 17–19 (1964).
Wyman, J. Measurements of the dielectric constants of conducting media. Phys. Rev. 35, 623–634 (1930).
Kirkwood, J. G. The dielectric polarization of polar liquids. J. Chem. Phys. 7, 911–919 (1939).
Fecko, C. J., Eaves, J. D., Loparo, J. J., Tokmakoff, A. & Geissler, P. L. Ultrafast hydrogen-bond dynamics in the infrared spectroscopy of water. Science 301, 1698–1702 (2003).
Bakker, H. J. & Skinner, J. L. Vibrational spectroscopy as a probe of structure and dynamics in liquid water. Chem. Rev. 110, 1498–1517 (2010).
Nihonyanagi, S. et al. Unified molecular view of the air/water interface based on experimental and theoretical χ(2) spectra of an isotopically diluted water surface. J. Am. Chem. Soc. 133, 16875–16880 (2011).
Du, Q., Freysz, E. & Shen, Y. R. Vibrational spectra of water molecules at quartz/water interfaces. Phys. Rev. Lett. 72, 238–241 (1994).
Schaefer, J., Gonella, G., Bonn, M. & Backus, E. H. G. Surface-specific vibrational spectroscopy of the water/silica interface: screening and interference. Phys. Chem. Chem. Phys. 19, 16875–16880 (2017).
Nihonyanagi, S., Yamaguchi, S. & Tahara, T. Direct evidence for orientational flip-flop of water molecules at charged interfaces: a heterodyne-detected vibrational sum frequency generation study. J. Chem. Phys. 130, 204704 (2009).
Wen, Y. C. et al. Unveiling microscopic structures of charged water interfaces by surface-specific vibrational spectroscopy. Phys. Rev. Lett. 116, 016101 (2016).
Nair, R. R. et al. Fine structure constant defines visual transparency of graphene. Science 320, 1308 (2008).
Ohto, T., Tada, H. & Nagata, Y. Structure and dynamics of water at water–graphene and water–hexagonal boron-nitride sheet interfaces revealed by ab initio sum-frequency generation spectroscopy. Phys. Chem. Chem. Phys. 20, 12979–12985 (2018).
Ostrowski, J. H. J. & Eaves, J. D. The tunable hydrophobic effect on electrically doped graphene. J. Phys. Chem. B 118, 530–536 (2014).
Du, Q., Superfine, R., Freysz, E. & Shen, Y. R. Vibrational spectroscopy of water at the vapor/water interface. Phys. Rev. Lett. 70, 2313–2316 (1993).
Stiopkin, I. V. et al. Hydrogen bonding at the water surface revealed by isotopic dilution spectroscopy. Nature 474, 192–195 (2011).
Ohno, P. E., Wang, H.-f., Paesani, F., Skinner, J. L. & Geiger, F. M. Second-order vibrational lineshapes from the air/water interface. J. Phys. Chem. A 122, 4457–4464 (2018).
Singla, S. et al. Insight on structure of water and ice next to graphene using surface-sensitive spectroscopy. ACS Nano 11, 4899–4906 (2017).
Ohno, P. E., Wang, H.-f. & Geiger, F. M. Second-order spectral lineshapes from charged interfaces. Nat. Commun. 8, 1032 (2017).
Gonella, G., Lütgebaucks, C., de Beer, A. G. F. & Roke, S. Second harmonic and sum-frequency generation from aqueous interfaces is modulated by interference. J. Phys. Chem. C 120, 9165–9173 (2016).
Ong, S., Zhao, X. & Eisenthal, K. B. Polarization of water molecules at a charged interface: second harmonic studies of the silica/water interface. Chem. Phys. Lett. 191, 327–335 (1992).
Zhang, Y., de Aguiar, H. B., Hynes, J. T. & Laage, D. Water structure, dynamics, and sum-frequency generation spectra at electrified graphene interfaces. J. Phys. Chem. Lett. 11, 624–631 (2020).
Bratko, D., Daub, C. D., Leung, K. & Luzar, A. Effect of field direction on electrowetting in a nanopore. J. Am. Chem. Soc. 129, 2504–2510 (2007).
von Domaros, M., Bratko, D., Kirchner, B. & Luzar, A. Dynamics at a Janus interface. J. Phys. Chem. C 117, 4561–4567 (2013).
Na, X., Ning, Z. & Rong-Qing, X. Effect of driving voltage polarity on dynamic response characteristics of electrowetting liquid lens. Jpn. J. Appl. Phys. 57, 052201 (2018).
Zhang, Y., Stirnemann, G., Hynes, J. T. & Laage, D. Water dynamics at electrified graphene interfaces: a jump model perspective. Phys. Chem. Chem. Phys. 22, 10581–10591 (2020).
Fumagalli, L. et al. Anomalously low dielectric constant of confined water. Science 360, 1339 (2018).
Alper, H. E. & Levy, R. M. Field strength dependence of dielectric saturation in liquid water. J. Phys. Chem. 94, 8401–8403 (1990).
Zhang, C. & Sprik, M. Electromechanics of the liquid water vapour interface. Phys. Chem. Chem. Phys. 22, 10676–10686 (2020).
Li, C.-Y. et al. In situ probing electrified interfacial water structures at atomically flat surfaces. Nat. Mater. 18, 697–701 (2019).
Benderskii, V. A. & Velichko, G. I. Temperature jump in electric double-layer study: Part I. Method of measurements. J. Electroanal. Chem. Interfacial Electrochem. 140, 1–22 (1982).
Kim, S. M. et al. The effect of copper pre-cleaning on graphene synthesis. Nanotechnology 24, 365602 (2013).
Shi, H. et al. Sensing local pH and ion concentration at graphene electrode surfaces using in situ Raman spectroscopy. Nanoscale 10, 2398–2403 (2018).
Chen, C.-C., Chang, C.-C., Li, Z., Levi, A. F. J. & Cronin, S. B. Gate tunable graphene–silicon ohmic/Schottky contacts. Appl. Phys. Lett. 101, 223113 (2012).
Stiopkin, I. V., Jayathilake, H. D., Weeraman, C. & Benderskii, A. V. Temporal effects on spectroscopic line shapes, resolution, and sensitivity of the broad-band sum frequency generation. J. Chem. Phys. 132, 234503 (2010).
Reina, A. et al. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 9, 30–35 (2009).
Das Sarma, S., Adam, S., Hwang, E. H. & Rossi, E. Electronic transport in two-dimensional graphene. Rev. Mod. Phys. 83, 407–470 (2011).
Froehlicher, G. & Berciaud, S. Raman spectroscopy of electrochemically gated graphene transistors: geometrical capacitance, electron–phonon, electron–electron, and electron–defect scattering. Phys. Rev. B 91, 205413 (2015).
Shi, H. et al. Monitoring local electric fields at electrode surfaces using surface enhanced Raman scattering-based Stark-shift spectroscopy during hydrogen evolution reactions. ACS Appl. Mater. Interfaces 10, 33678–33683 (2018).
Sethna, P. P., Palmer, K. F. & Williams, D. Optical constants of D2O in the infrared. J. Opt. Soc. Am. 68, 815–817 (1978); https://doi.org/10.1364/JOSA.68.000815.
Joutsuka, T., Hirano, T., Sprik, M. & Morita, A. Effects of third-order susceptibility in sum frequency generation spectra: a molecular dynamics study in liquid water. Phys. Chem. Chem. Phys. 20, 3040–3053 (2018).
Acknowledgements
This research was supported by Air Force Office of Scientific Research grant nos FA9550-15-1-0184 and FA9550-19-1-0115 (A.M., C.D., M.M., S.B.C., A.V.B.), Army Research Office award no. W911NF-17-1-0325 (B.Z.), US Department of Energy, Office of Science, Office of Basic Energy Sciences award DE-SC0019322 (B.H.), and National Science Foundation award no. CHE-1708581 (H.S.).
Author information
Authors and Affiliations
Contributions
H.S., B.H., B.Z. and S.B.C. manufactured graphene electrodes. A.M., H.S., B.H. and S.B.C. performed electrochemical measurements and Raman spectroscopy. A.M., C.D., M.M., D.B. and A.V.B. performed VSFG measurements. A.M., C.D., M.M. and A.V.B. contributed VSFG spectral analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Franz Geiger, Dusan Bratko, Poul Petersen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Raman spectrum of the graphene electrode.
For a high-quality, defect-free graphene monolayer, the ratio of intensities of the 2D to G band is about 2. A.U., arbitrary units.
Extended Data Fig. 2 Electrochemical current with D2O in the cell versus applied voltage to the graphene electrode versus Ag/AgCl.
The fact that the current magnitude does not exceed 100 μA for the voltages that were applied implies that water splitting does not occur to an appreciable degree.
Extended Data Fig. 3 SFG spectra of the graphene–D2O interface at 0.3 V (versus Ag/AgCl).
Spectra at 0.3 V were taken several times throughout the experiment to verify that there was no drift in the SFG signal with time.
Extended Data Fig. 4 SFG spectra of graphene–D2O following dilution with H2O.
Isotopic exchange weakens the peak at ~2,700 cm−1, confirming that D2O is responsible for this signal. A.U., arbitrary units.
Extended Data Fig. 5 Linear dependence of G-band Raman shift on applied voltage in both the two-terminal and three-terminal configurations.
A two-terminal voltage (applied versus glassy carbon) can be converted to a voltage versus Ag/Cl in the three-terminal configuration by exploiting the linearity of the G-band shift with respect to the applied voltage.
Extended Data Fig. 6 Electric-field-dependent SFG spectra of the graphene–D2O interface.
a–c, Spectra are shown for the graphene–D2O interface (a), and its decomposition into the \({\chi }_{{\rm{s}}}^{(2)}\) (surface; b) and \({\chi }_{{\rm{S}},{\rm{DL}}}^{(2)}\,\) (bulk; c) contributions. With the bulk contribution known from the literature, the surface contribution is found through a spectral fitting procedure.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Montenegro, A., Dutta, C., Mammetkuliev, M. et al. Asymmetric response of interfacial water to applied electric fields. Nature 594, 62–65 (2021). https://doi.org/10.1038/s41586-021-03504-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03504-4
This article is cited by
-
Impact of the aqueous corrosion induced alteration layer on mechanical properties of pharmaceutical glasses
npj Materials Degradation (2024)
-
Ab initio theory of the nonequilibrium adsorption energy
npj Computational Materials (2024)
-
Hydration lubrication modulated by water structure at TiO2-aqueous interfaces
Friction (2024)
-
Exploring the Cation Regulation Mechanism for Interfacial Water Involved in the Hydrogen Evolution Reaction by In Situ Raman Spectroscopy
Nano-Micro Letters (2024)
-
Chemistry governs water organization at a graphene electrode
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.