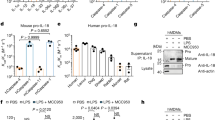
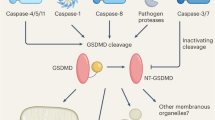
Abstract
As organelles of the innate immune system, inflammasomes activate caspase-1 and other inflammatory caspases that cleave gasdermin D (GSDMD). Caspase-1 also cleaves inactive precursors of the interleukin (IL)-1 family to generate mature cytokines such as IL-1β and IL-18. Cleaved GSDMD forms transmembrane pores to enable the release of IL-1 and to drive cell lysis through pyroptosis1,2,3,4,5,6,7,8,9. Here we report cryo-electron microscopy structures of the pore and the prepore of GSDMD. These structures reveal the different conformations of the two states, as well as extensive membrane-binding elements including a hydrophobic anchor and three positively charged patches. The GSDMD pore conduit is predominantly negatively charged. By contrast, IL-1 precursors have an acidic domain that is proteolytically removed by caspase-110. When permeabilized by GSDMD pores, unlysed liposomes release positively charged and neutral cargoes faster than negatively charged cargoes of similar sizes, and the pores favour the passage of IL-1β and IL-18 over that of their precursors. Consistent with these findings, living—but not pyroptotic—macrophages preferentially release mature IL-1β upon perforation by GSDMD. Mutation of the acidic residues of GSDMD compromises this preference, hindering intracellular retention of the precursor and secretion of the mature cytokine. The GSDMD pore therefore mediates IL-1 release by electrostatic filtering, which suggests the importance of charge in addition to size in the transport of cargoes across this large channel.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Atomic coordinates of the 33-fold symmetric human GSDMD pore structure have been deposited in the PDB under accession number 6VFE. The cryo-EM density maps of the 33-fold symmetric pore and the prepore have been deposited in the Electron Microscopy Data Bank under accession numbers EMD-21160 and EMD-21161, respectively. All other data are available from the corresponding authors upon reasonable request.
References
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Aglietti, R. A. et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. USA 113, 7858–7863 (2016).
Sborgi, L. et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 35, 1766–1778 (2016).
Evavold, C. L. et al. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44.e6 (2018).
Heilig, R. et al. The gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur. J. Immunol. 48, 584–592 (2018).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021).
Monteleone, M. et al. Interleukin-1β maturation triggers its relocation to the plasma membrane for gasdermin-D-dependent and -independent secretion. Cell Rep. 24, 1425–1433 (2018).
Liu, X., Xia, S., Zhang, Z., Wu, H. & Lieberman, J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov. (2021).
Orning, P. et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 (2018).
Sarhan, J. et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. USA 115, E10888–E10897 (2018).
Burgener, S. S. et al. Cathepsin G inhibition by Serpinb1 and Serpinb6 prevents programmed necrosis in neutrophils and monocytes and reduces GSDMD-driven inflammation. Cell Rep. 27, 3646–3656.e5 (2019).
Zhang, Z. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020).
Zhou, Z. et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, eaaz7548 (2020).
Sollberger, G. et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 3, eaar6689 (2018).
Wang, Q. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579, 421–426 (2020).
Chen, K. W. et al. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 3, eaar6676 (2018).
Ruan, J., Xia, S., Liu, X., Lieberman, J. & Wu, H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67 (2018).
Mulvihill, E. et al. Mechanism of membrane pore formation by human gasdermin-D. EMBO J. 37, e98321 (2018).
Liu, Z. et al. Crystal structures of the full-length murine and human gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity 51, 43–49.e4 (2019).
Rühl, S. et al. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960 (2018).
Lee, I. H., Kai, H., Carlson, L. A., Groves, J. T. & Hurley, J. H. Negative membrane curvature catalyzes nucleation of endosomal sorting complex required for transport (ESCRT)-III assembly. Proc. Natl Acad. Sci. USA 112, 15892–15897 (2015).
Davis, M. A. et al. Calpain drives pyroptotic vimentin cleavage, intermediate filament loss, and cell rupture that mediates immunostimulation. Proc. Natl Acad. Sci. USA 116, 5061–5070 (2019).
Rogers, C. et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 10, 1689 (2019).
van Pee, K. et al. CryoEM structures of membrane pore and prepore complex reveal cytolytic mechanism of Pneumolysin. eLife 6, e23644 (2017).
Tapia, V. S. et al. The three cytokines IL-1β, IL-18, and IL-1α share related but distinct secretory routes. J. Biol. Chem. 294, 8325–8335 (2019).
Martín-Sánchez, F. et al. Inflammasome-dependent IL-1β release depends upon membrane permeabilisation. Cell Death Differ. 23, 1219–1231 (2016).
Batista, S. J. et al. Gasdermin-D-dependent IL-1α release from microglia promotes protective immunity during chronic Toxoplasma gondii infection. Nat. Commun. 11, 3687 (2020).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl Acad. Sci. USA 98, 10037–10041 (2001).
Xia, S., Ruan, J. & Wu, H. Monitoring gasdermin pore formation in vitro. Methods Enzymol. 625, 95–107 (2019).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Acknowledgements
We thank B. Honig, T. Rapoport, F. Shao, J. Kagan, D. Golenbock, S. Blacklow, A. Kruse and M. Liao for discussions. For structural data collection, we thank R. Walsh, S. Sterling, S. Rawson and Z. Li at the Harvard Cryo-EM Center for Structural Biology and K. Song, K. Lee and C. Xu at the Cryo-EM Core Facility at University of Massachusetts Medical School. This work was supported by US National Institutes of Health grants R01AI139914 (H.W. and J.L.), DP1HD087988 (H.W.), R01AI124491 (H.W.), R01CA240955 (J.L.), R01DK095045 (A.G.), R01DK099465 (A.G.) and 5T32HL066987-18 (T.-M.F.). S.X. received an Albert J. Ryan fellowship. J.R. and Z.Z. received postdoctoral fellowships from the Charles A. King Trust.
Author information
Authors and Affiliations
Contributions
H.W., J.R. and S.X. conceived the study. S.X. and J.R. reconstituted and optimized GSDMD assemblies and determined the cryo-EM structures. S.X., Z.Z. and V.G.M. performed cellular experiments. S.X., J.L.P., Y.D., S.M.V., L.W. and T.-M.F. performed biochemical experiments. H.W., J.L., A.G. and M.P.J. supervised the project. All authors organized and analysed data. H.W. and S.X. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
H.W. and J.L. are co-founders of Ventus Therapeutics. The other authors declare no competing interests.
Additional information
Peer review information Nature thanks Helen Saibil and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Reconstitution and purification of GSDMD assemblies.
a, Optimized construct for human GSDMD, referred to as wild-type (WT) GSDMD for convenience. The N-terminal MBP tag and the TEV-cleavable linker between MBP and GSDMD-NT are not shown. b, Schematic of GSDMD pore and prepore reconstitution. c, Reduced, but not abolished, activity of the GSDMD(L192E) mutant shown by Tb3+ leakage assay (n = 3 biological replicates). En, activating enzyme. d, e, Size-exclusion chromatography profiles (d) and an enlargement of the boxed region (e). The box encloses the fractions containing the majority of wild-type GSDMD or GSDMD(L192E) assemblies. The shaded fractions containing well-dispersed particles (e) were used for electron microscopy data collection. f, SDS–PAGE showing wild-type GSDMD-NT at around 30 kDa from the corresponding fractions in e. g, Detergent screen. A group of non-ionic detergents with commercial shorthand CxEy (Cx, x number of carbons in the alkyl chain; Ey, y number of ethylene glycol repeats) yielded stable GSDMD pores. C12E8 was chosen as the final solubilizing agent. Scale bars, 200 nm. h, GSDMD pores extracted by 1% C12E8 from liposomes containing different types and amounts (%) of acidic lipids. Liposomes containing 20% PA were chosen. Scale bars, 200 nm. i, Sizes of GSDMD and GSDMA3 assemblies reconstituted on liposomes containing different acidic lipids (20%) and extracted by different types of detergent (1% CxEy, or 50 mM cholate), shown by outer diameters measured under negative-stain electron microscopy (from left to right, n = 64, 42, 77, 73, 14, 34, 45, 23, 21 and 18 particles). Data shown in c and i are mean ± s.d. Data shown in f–h are representative of three independent experiments.
Extended Data Fig. 2 Cryo-EM data processing for the wild-type GSDMD dataset.
a, A cryo-EM image of the wild-type GSDMD sample. Data are representative of three independent experiments. Scale bar, 100 nm. b, Processing of the wild-type GSDMD cryo-EM dataset. Initial 2D classes showed a ring-stacking phenomenon, which added to structural heterogeneity and posed challenges for symmetry determination. Density subtraction was therefore performed, followed by 3D reconstruction of each ring without assumption of symmetry, after which particle symmetry could be determined for certain 3D classes. These classes were then refined with their respective symmetry imposed to yield final cryo-EM maps.
Extended Data Fig. 3 Cryo-EM data processing for the GSDMD(L192E) dataset.
a, A cryo-EM image of the GSDMD(L192E) sample. Data are representative of three independent experiments. Scale bar, 100 nm. b, Processing of the GSDMD(L192E) cryo-EM dataset. This dataset was first processed following the procedures for the wild-type dataset. Cryo-EM maps obtained from 3D refinement with symmetry imposed were further classified without alignment to remove heterogeneous particles. Then, the best 3D classes were refined again to improve resolutions. A 3D reconstruction at 7.3 Å was further improved by symmetry expansion, 3D classification without alignment, 3D local refinement, and per-particle CTF refinement to reach the final map at 3.9 Å resolution. c, Similarity of cryo-EM maps generated from the wild-type and L192E datasets. Owing to the higher resolutions, maps from the L192E dataset were chosen for model building.
Extended Data Fig. 4 Analysis of cryo-EM densities and models.
a, Half-map-to-half-map and model-to-map FSC for the 33-fold symmetric GSDMD pore and prepore from the L192E dataset. Horizontal dashed lines represent FSC cut-offs at 0.5 and 0.143. b, c, Pore-form (b) and prepore-form (c) GSDMD subunits fitted into their respective cryo-EM density. Arrows indicate secondary structural elements specified by residue numbers. d, Close-up views of the pore-form GSDMD structure fitted into its cryo-EM density at six representative locations denoted by residue numbers.
Extended Data Fig. 5 β-hairpin extension and prepore-to-pore transition.
a, Comparison between autoinhibited, prepore-form and pore-form GSDMD. The autoinhibited GSDMD-NT was obtained from the crystal structure of full-length GSDMD (PDB: 6N9O). The β4 strand and α4 helix are invisible in the crystal structure and were modelled on the basis of the crystal structure of full-length GSDMA3 (PDB: 5B5R). b, Formation of β-hairpins. The β3–β4–β5 region constitutes the first extension domain (ED1), which transforms into hairpin (HP) 1 by refolding. The β7–α4–β8 region represents ED2 and becomes HP2. c, Sequence alignment of human and mouse GSDMD, with secondary structures and key residues denoted. Blue highlighting indicates residues responsible for lipid binding, through either hydrophobic or charged interactions; green highlighting indicates residues at inter-subunit interfaces; and underlining indicates residues that are important for membrane insertion. d, Conserved rigid-body movement of the globular domain (‘palm’) towards the membrane-distal direction during GSDM pore formation, shown by alignment of the GSDMA3 pore structure (PDB: 6CB8) and prepore model at their central axes.
Extended Data Fig. 6 Hydrophobic anchor and basic patches of GSDMs.
a, Effects of mutations in the hydrophobic anchor on GSDMD pore formation assessed by Tb3+ leakage from liposomes (n = 3 biological replicates). b, GSDM sequences aligned at the hydrophobic anchor and BPs. Blue highlighting indicates basic residues at BPs; green highlighting indicates hydrophobic residues of the anchor; and dashes indicate gaps. c, The GSDMA3 prepore model with the β1–β2 loop highlighted in green and BP1 shown in blue. A GSDMA3 prepore subunit is also shown in two orientations. d, A side view of pore-form GSDMA3 (PDB: 6CB8), with electrostatic surface shown around the β1–β2 loop. The anchor and BP2 are boxed in green. e, Impairment of the pore-forming ability of GSDMA3 by mutations in the hydrophobic anchor, shown by Tb3+ leakage assay (n = 3 biological replicates). ‘Anchor’ indicates that L47, F48 and W49 are mutated to E. f, A cryo-EM density blob that probably represents heads of phospholipids near BP3. Basic residues in BP3 point towards the blob. g, Effects of mutations in BP1 (R7, R10 and R11 mutated to E), BP2 (R42, K43, K51 and R53 mutated to E) and BP3 (K204 or R174 mutated to E) on GSDMD activity evaluated by Tb3+ leakage assay (n = 3 biological replicates). h, Importance of BP2 for GSDMA3 pore formation, shown by Tb3+ leakage assay (n = 3 biological replicates). Here, in BP2, R41, K42, R43 and K44 are mutated to E. i, Exposure of the hydrophobic anchor and BP2 upon removal of the inter-domain linker between GSDMD-NT and GSDMD-CT. Surface representations are shown for autoinhibited GSDMD (PDB: 6N9O) with and without the inferred linker (cyan curve connecting Q241 and T284). Purple, GSDMD-NT; black: GSDMD-CT; green, anchor–BP2 region; yellow, two ends of the linker. Data shown in a, e, g and h are mean ± s.d.
Extended Data Fig. 7 GSDM acidic patches and their mutations.
a, Locations of APs shown by aligned GSDM sequences. Dots, strings of omitted uncharged residues; red highlighting, acidic residues; blue, basic residues. Of note, the basic residues near the APs may face the membrane (such as those in BP3) and therefore do not necessarily weaken the acidity of the pore conduit. b, Assessment of alanine mutations of GSDMD APs 1–4 by Tb3+ leakage assay (n = 3 biological replicates). c, Assessment of alanine mutations of GSDMA3 AP1 and AP2 by Tb3+ liposome leakage assay (n = 3 biological replicates). d, Negative-staining electron microscopy images of wild-type and AP-mutant GSDMD and GSDMA3 assemblies, solubilized from liposomes using C12E8 and cholate, respectively. Data shown are representative of three independent experiments. Scale bars, 100 nm. e, Outer diameters of wild-type or AP-mutant GSDMD and GSDMA3 assemblies, measured under negative-staining electron microscopy (n = 50 particles per group). Data shown in b, c and e are mean ± s.d.
Extended Data Fig. 8 Liposome experiments and electrostatics analysis.
a, Unlysed liposomes (25–75% PS) demonstrate that LDH release is minimal when GSDMD is added at a sub-lytic concentration (1× = 0.5 μM) (n = 3 biological replicates). b, Release of cyt c, CRYGD and OCM from liposomes permeabilized by GSDMD shown by immunoblotting. c, Similar rates of GSDMD pore formation on liposomes of various acidic lipid contents (25–75% PS), according to Tb3+ release assay (n = 3 biological replicates). d, Preferential IL-1β release from liposomes (50% and 75% PS) perforated by GSDMD shown by immunoblotting. e, Release of pro-IL-18 and IL-18 from liposomes permeabilized by GSDMD shown by immunoblotting. f, Minimal LDH release when GSDMA3 was activated at a sub-lytic concentration (1× = 0.5 μM) (n = 3 biological replicates), demonstrating that the liposomes are intact. g, Release of pro-IL-1β and mature IL-1β from liposomes perforated by GSDMA3 shown by immunoblotting. h, Release rates of IL-1β cargoes through GSDMD pores shown by fitted hyperbolic functions. i, Initial release rates (r) extrapolated from h. j, Charge differences among the cargoes (∆q) and rate ratios (R). k, Plot of ln(R) against ∆q to estimate the electrostatic potential (E) of the GSDMD pore conduit. l, A lack of release of encapsulated bulky FITC-labelled dextrans (2 MDa) when SLO or PFO was added at a sub-lytic concentration (1× = 0.1 μM) (n = 3 biological replicates), demonstrating that the liposomes are intact. m, Similar release of pro-IL-1β and mature IL-1β from liposomes permeabilized by PFO. n, Electrostatic surfaces of the modelled PFO pore conduit. Data shown in a, c, f and l are mean ± s.d. Data are representative of three (b) or two (d, e, g, m) independent experiments.
Extended Data Fig. 9 Macrophage experiments.
a, Comparable expression of wild-type and charge-mutant GSDMD in GSDMD-knockout iBMDMs, and of wild-type and charge-mutant pro-IL-1β in IL-1β-knockout iBMDMs, shown by immunoblotting. b, c, Similar sensitivity of knockout iBMDMs reconstituted with wild-type or charge-mutant GSDMD or pro-IL-1β to pyroptosis and death evasion by glycine protection (b) or low-dose nigericin treatment (c), shown by LDH release (n = 3 biological replicates). d, Cleavage of engineered GSDMA3 chimera (A3chim) by caspase-1 (C1), shown by SDS–PAGE of proteolysis reactions using purified proteins. e, Comparable expression of A3chim (Flag-tagged) and its AP mutants in GSDMD-knockout cells. AP1, 4 D/E to A; AP2, 2 D/E to A. f, g, Preferential release of mature IL-1β from glycine-protected living iBMDMs permeabilized by A3chim, shown by immunoblotting (f) and LDH release (n = 3 biological replicates) (g). h, i, IL-1β release from GSDMD-knockout iBMDMs expressing wild-type or AP-mutant A3chim under glycine protection, shown by immunoblotting (h) and LDH release (n = 3 biological replicates) (i). j–l, IL-1β release from living GSDMD-knockout iBMDMs expressing A3chim stimulated by low-dose nigericin, characterized by immunoblotting (j), LDH release (n = 3 biological replicates) (k) and ELISA (n = 3 biological replicates) (l). m, n, IL-1β release from low-dose nigericin-stimulated GSDMD-knockout iBMDMs expressing wild-type or AP-mutant A3chim, evaluated by immunoblotting (m), LDH release (n = 3 biological replicates) (n) and ELISA (n = 3 biological replicates) (o). Data shown in b, c, g, i, k, l, n and o are mean ± s.d. Data shown in a, d–f, h, j and m are representative of two independent experiments.
Supplementary information
Supplementary Figure 1
Uncropped SDS-PAGE gels and immunoblots. Relative positions of the scans within a panel (black box) follow those in the figures. Blue boxes indicate cropped areas. Molecular weight markers are shown in kDa.
Video 1
The GSDMD prepore-to-pore transition The morph simulation was generated by overlaying structures of the 33-subunit prepore and pore at their α1 helices. During pore formation, the globular domain rotates away from the membrane while the β-barrel inserts into the membrane.
Video 2
Conformational changes of a single GSDMD subunit In addition to the formation and insertion of the β-hairpins, a rotational movement away from the membrane of the whole globular domain can be observed.
Rights and permissions
About this article
Cite this article
Xia, S., Zhang, Z., Magupalli, V.G. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021). https://doi.org/10.1038/s41586-021-03478-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03478-3
This article is cited by
-
Biological and clinical roles of IL-18 in inflammatory diseases
Nature Reviews Rheumatology (2024)
-
Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets
Nature Reviews Drug Discovery (2024)
-
NINJ1 induces plasma membrane rupture and release of damage-associated molecular pattern molecules during ferroptosis
The EMBO Journal (2024)
-
The gasdermin family: emerging therapeutic targets in diseases
Signal Transduction and Targeted Therapy (2024)
-
Crosstalk of HDAC4, PP1, and GSDMD in controlling pyroptosis
Cell Death & Disease (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.