Abstract
Chronic, sustained exposure to stressors can profoundly affect tissue homeostasis, although the mechanisms by which these changes occur are largely unknown. Here we report that the stress hormone corticosterone—which is derived from the adrenal gland and is the rodent equivalent of cortisol in humans—regulates hair follicle stem cell (HFSC) quiescence and hair growth in mice. In the absence of systemic corticosterone, HFSCs enter substantially more rounds of the regeneration cycle throughout life. Conversely, under chronic stress, increased levels of corticosterone prolong HFSC quiescence and maintain hair follicles in an extended resting phase. Mechanistically, corticosterone acts on the dermal papillae to suppress the expression of Gas6, a gene that encodes the secreted factor growth arrest specific 6. Restoring Gas6 expression overcomes the stress-induced inhibition of HFSC activation and hair growth. Our work identifies corticosterone as a systemic inhibitor of HFSC activity through its effect on the niche, and demonstrates that the removal of such inhibition drives HFSCs into frequent regeneration cycles, with no observable defects in the long-term.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) with the accession code GSE135705. The DAVID web-accessible tool (v.6.8) is available at https://david.ncifcrf.gov/. MetazSecKB web-accessible tool is available at proteomics.ysu.edu/secretomes/animal/. Source data are provided with this paper.
References
Shwartz, Y. et al. Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 182, 578–593.e19 (2020).
Lay, K., Kume, T. & Fuchs, E. FOXC1 maintains the hair follicle stem cell niche and governs stem cell quiescence to preserve long-term tissue-regenerating potential. Proc. Natl Acad. Sci. USA 113, E1506–E1515 (2016).
Wang, L., Siegenthaler, J. A., Dowell, R. D. & Yi, R. Foxc1 reinforces quiescence in self-renewing hair follicle stem cells. Science 351, 613–617 (2016).
Plikus, M. V. et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451, 340–344 (2008).
Müller-Röver, S. et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117, 3–15 (2001).
Greco, V. et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4, 155–169 (2009).
Hsu, Y. C., Li, L. & Fuchs, E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 157, 935–949 (2014).
Sawaya, M. E. & Hordinsky, M. K. Glucocorticoid regulation of hair growth in alopecia areata. J. Invest. Dermatol. 104, 30S (1995).
Stenn, K. S., Paus, R., Dutton, T. & Sarba, B. Glucocorticoid effect on hair growth initiation: a reconsideration. Skin Pharmacol. 6, 125–134 (1993).
Pérez, P. et al. Altered skin development and impaired proliferative and inflammatory responses in transgenic mice overexpressing the glucocorticoid receptor. FASEB J. 15, 2030–2032 (2001).
Rose, J. & Sterner, M. The role of the adrenal glands in regulating onset of winter fur growth in mink (Mustela vison). J. Exp. Zool. 262, 469–473 (1992).
Butcher, E. O. Hair growth in adrenalectomized, and, adrenalectomized thyroxin-treated rats. Am. J. Physiol. 120, 427–434 (1937).
Whiteley, H. J. The effect of adrenalectomy and adrenocortical hormones on the hair growth cycle in the rabbit and rat. J. Endocrinol. 17, 167–176 (1958).
Hsu, Y. C., Pasolli, H. A. & Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144, 92–105 (2011).
Rompolas, P., Mesa, K. R. & Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502, 513–518 (2013).
Chen, C. C. et al. Regenerative hair waves in aging mice and extra-follicular modulators follistatin, Dkk1, and Sfrp4. J. Invest. Dermatol. 134, 2086–2096 (2014).
Keyes, B. E. et al. Nfatc1 orchestrates aging in hair follicle stem cells. Proc. Natl Acad. Sci. USA 110, E4950–E4959 (2013).
Walczak, E. M. & Hammer, G. D. Regulation of the adrenocortical stem cell niche: implications for disease. Nat. Rev. Endocrinol. 11, 14–28 (2015).
Besnard, A. et al. Targeting Kruppel-like factor 9 in excitatory neurons protects against chronic stress-induced impairments in dendritic spines and fear responses. Cell Rep. 23, 3183–3196 (2018).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
Tye, K. M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013).
Enshell-Seijffers, D., Lindon, C., Kashiwagi, M. & Morgan, B. A. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 18, 633–642 (2010).
Festa, E. et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146, 761–771 (2011).
Zhang, B. et al. Hair follicles’ transit-amplifying cells govern concurrent dermal adipocyte production through Sonic Hedgehog. Genes Dev. 30, 2325–2338 (2016).
Clavel, C. et al. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev. Cell 23, 981–994 (2012).
Driskell, R. R., Giangreco, A., Jensen, K. B., Mulder, K. W. & Watt, F. M. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 136, 2815–2823 (2009).
Horsley, V., Aliprantis, A. O., Polak, L., Glimcher, L. H. & Fuchs, E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132, 299–310 (2008).
Rhee, H., Polak, L. & Fuchs, E. Lhx2 maintains stem cell character in hair follicles. Science 312, 1946–1949 (2006).
Leishman, E. et al. Foxp1 maintains hair follicle stem cell quiescence through regulation of Fgf18. Development 140, 3809–3818 (2013).
Choi, Y. S. et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 13, 720–733 (2013).
Rothlin, C. V., Carrera-Silva, E. A., Bosurgi, L. & Ghosh, S. TAM receptor signaling in immune homeostasis. Annu. Rev. Immunol. 33, 355–391 (2015).
Wang, Y. et al. Axl-altered microRNAs regulate tumorigenicity and gefitinib resistance in lung cancer. Cell Death Dis. 5, e1227 (2014).
Asiedu, M. K. et al. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene 33, 1316–1324 (2014).
Balaji, K. et al. AXL inhibition suppresses the DNA damage response and sensitizes cells to PARP inhibition in multiple cancers. Mol. Cancer Res. 15, 45–58 (2017).
Aoki, E., Shibasaki, T. & Kawana, S. Intermittent foot shock stress prolongs the telogen stage in the hair cycle of mice. Exp. Dermatol. 12, 371–377 (2003).
Zhang, B. et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 577, 676–681 (2020).
Arck, P. C. et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am. J. Pathol. 162, 803–814 (2003).
Mittelstadt, P. R., Monteiro, J. P. & Ashwell, J. D. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J. Clin. Invest. 122, 2384–2394 (2012).
Morris, R. J. et al. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22, 411–417 (2004).
Kang, S. H., Fukaya, M., Yang, J. K., Rothstein, J. D. & Bergles, D. E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668–681 (2010).
Arnold, K. et al. Sox2+ adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9, 317–329 (2011).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Holland, S. J. et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 70, 1544–1554 (2010).
Goldstein, J. M. et al. In situ modification of tissue stem and progenitor cell genomes. Cell Rep. 27, 1254–1264.e7 (2019).
Plikus, M. V. & Chuong, C. M. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J. Invest. Dermatol. 128, 1071–1080 (2008).
Rezza, A. et al. Signaling networks among stem cell precursors, transit-amplifying progenitors, and their niche in developing hair follicles. Cell Rep. 14, 3001–3018 (2016).
Rendl, M., Lewis, L. & Fuchs, E. Molecular dissection of mesenchymal–epithelial interactions in the hair follicle. PLoS Biol. 3, e331 (2005).
Joost, S. et al. The molecular anatomy of mouse skin during hair growth and rest. Cell Stem Cell 26, 441–457.e7 (2020).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Käll, L., Krogh, A. & Sonnhammer, E. L. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 338, 1027–1036 (2004).
Meinken, J., Walker, G., Cooper, C. R. & Min, X. J. MetazSecKB: the human and animal secretome and subcellular proteome knowledgebase. Database (Oxford) 2015, bav077 (2015).
Nowak, J. A. & Fuchs, E. Isolation and culture of epithelial stem cells. Methods Mol. Biol. 482, 215–232 (2009).
Acknowledgements
We thank many colleagues who donated mice to The Jackson Laboratory; A. Regev, Y. Fong, and members of the Hsu laboratory—in particular Y. Shwartz—for discussions and comments on the manuscript, and O. Chung for technical assistance; and HCBI, HSCRB-HSCI FACS core, HSCRB Histology core, Small Molecule Mass Spectrometry Facility, Office of Animal Resources, and the Bauer Core Sequencing Facility at Harvard University for technical support. This work was supported in part by the New York Stem Cell Foundation (Y.-C.H.); the Smith Family Foundation Odyssey Award (Y.-C.H.); the Pew Charitable Trusts (Y.-C.H.); the Harvard Stem Cell Institute (Y.-C.H. and A.S.); a Harvard HMS Dean’s Award (Y.-C.H.); the American Cancer Society (Y.-C.H.); a James and Audrey Foster MGH Research Scholar Award (A.S.); a NARSAD Young Investigator Award (A.B.); MGH ECOR Fund for Medical Discovery Postdoctoral Fellowship Awards (A.B.); the New York State Department of Health (NYSTEM-C029574, NYSTEM-C32561GG to M.R.); NINDS (R56NS117529 to A.S.) and NIH (R01-AR070825 to Y.-C.H; R35-HL139598 to M.N.; R01MH104175, R01AG048908 and 1R01MH111729 to A.S.; R01AR071047 and R01AR063151 to M.R.). Y.-C.H. is a New York Stem Cell Foundation – Robertson Investigator and a Pew Scholar. J.D.B. and S.M. acknowledge support from the Broad Institute Fellows Program. X.J. is a Junior Fellow of, and is supported by, the Society of Fellows of Harvard University.
Author information
Authors and Affiliations
Contributions
Y.-C.H. and S.C. conceived the project. S.C. performed most of the experiments. B.Z. and S.M. performed bioinformatic analysis. M.G.-C., D.S. and S.T.K. performed experiments related to chronic unpredictable stress, corticosterone feeding and adrenalectomy. X.J. and Y.-L.K. generated RNA-seq libraries. A.R. and L.G. performed GR RT–qPCR for DP and DF samples. B.Z., J.D.B., M.R., M.N., A.S. and A.B. provided intellectual input. Y.-C.H. and S.C. wrote the manuscript, with discussion and feedback from all co-authors.
Corresponding author
Ethics declarations
Competing interests
A patent application covering the methods and compositions for controlling hair growth has been filed by the President and Fellows of Harvard College, listing Y.-C.H. and S.C. as inventors. The other authors declare no competing interests related to this work.
Additional information
Peer review information Nature thanks Eduardo Leonardo, William Lowry, Rui Yi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Hair cycle progression in ADX mice over time.
a, Hair cycle with immunohistochemical analyses (PCAD) in sham and ADX mice. b, Hair cycle progression in sham male and ADX male mice. c, Schematic depicting HFSCs in anagen and telogen. The upper ORS of anagen hair follicles contributes to the new bulge and hair germ (HG) of the following telogen hair follicles. See refs. 14,15 for details. d, The ORS length in the zigzag hairs of sham (P113) and ADX (P65) mice during late anagen. The brackets indicate the ORS length below the bulge. e, The hair shaft length of each hair subtype in sham and ADX mice after anagen. f, H&E staining at P65 of skin from sham and ADX mice. g, Immunohistochemical analyses (Sox9 and CD34) in telogen (Telo), late anagen (AnaV), and mid catagen (CatV) hair follicles. Yellow dashed lines, bulge; white dashed lines, HG (telo), hair follicle (AnaV, CatV); solid line, DP. h, Immunocolocalization (EdU and CD34) in infundibulum (IF), junctional zone (JZ), sebaceous gland (SG), mid ORS (ORSmid), lower ORS (ORSlow) and matrix (Mx) of late anagen (AnaVI) hair follicles. The dashed lines outline the hair follicle. i, Left, H&E staining in the late anagen skin of sham and ADX mice with quantification of the epidermal thickness (E). Right, immunocolocalization (EdU and DAPI) in interfollicular epidermis (IFE) and dermis. Dashed lines indicate the boundary between the epidermis and the dermis. j, Representative hair regrowth status of sham and ADX mice from P60 to P549. k, Duration of telogen in sham and ADX mice. l, H&E staining of skin from young sham, aged sham, and aged ADX mice with quantification of the number of hair follicles per mm. Yellow dashed lines, bulge; white dashed lines, HG, solid lines, DP. Scale bars, 50 μm (a, d, f–i, l), 1 mm (e). Data are mean ± s.e.m. *P < 0.05, ****P < 0.0001, NS, not significant. For exact P values, see Source Data. For statistics, sample sizes and numbers of replications, see Methods.
Extended Data Fig. 2 Corticosterone restores normal hair cycle progression in ADX mice.
a, H&E staining of the skin of 21-month-old sham and ADX mice. b, Morphology of each hair subtype from the skin of 18-month-old sham and ADX mice. c, Left, immunohistochemical analyses (CD34 and PCAD) of telogen hair follicles in the skin of sham and ADX mice at 22 months old, showing normal hair follicle morphology and comparable stem-cell numbers. Middle, quantification of the number of bulge and hair germ cells per HF. Right, the percentage of HFSCs in epithelial fraction by FACS. d, Hormones from the adrenal gland and plasma levels of corticosterone in P45 sham and ADX mice. e, Plasma levels of noradrenaline and adrenaline measured by LC–MS/MS at P45 (10 days after surgery) in sham and ADX mice. f, Left, experimental design to test if supplying corticosterone rescues ADX phenotypes. Right, hair cycle progression of sham mice fed with vehicle (sham+veh) or ADX mice fed with corticosterone (ADX+CORT). g, Plasma corticosterone levels at P62 in C57BL/6 mice after a week’s feeding with vehicle or corticosterone. h, Top, experimental design for 3 days of corticosterone feeding. Bottom, the percentage of hair regrowth of the back skin at P38. i, Hair cycle progression of C57BL/6 male mice fed with vehicle or corticosterone. Corticosterone feeding prolonged telogen as long as corticosterone was provided to the mice (both male and female). j, Body weight of C57BL/6 mice fed with vehicle or corticosterone from P83 to P118. k, Left, H&E staining in the skin of vehicle and corticosterone-fed mice. Middle and right, quantification of the thickness of dermis (middle) and dermal adipose layer (right). D, dermis; A, adipose layer. l, Immunohistochemical analysis (active caspase 3 (aCAS3) and PCAD) in vehicle- and corticosterone-fed mice. Dashed lines, epidermis and hair follicles. m, Left, experimental design to test the effect of corticosterone withdrawal. Right, hair cycle progression of C57BL/6 mice after completion of 3 weeks of vehicle or corticosterone feeding. Scale bars (a, b, c, k, l), 50 μm. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS, not significant. For exact P values, see Source Data. For statistics, sample sizes and numbers of replications, see Methods.
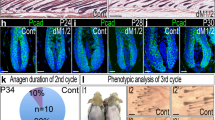
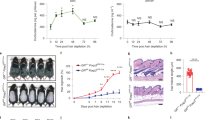
Extended Data Fig. 3 Removal of the adrenal glands in stressed or aged mice leads to hair follicle regeneration.
a, Plasma corticosterone levels at P62 in non-stressed control and stressed mice. b, c, H&E staining (b) and immunohistochemical analyses (active caspase3 (aCAS3) and PCAD) (c) in control and stressed mice. Dashed lines, epidermis and hair follicles. d, Stressed sham (sham+stress) and stressed ADX (ADX+stress) mice were monitored for hair coat recovery. Quantification shows the percentage of back skin that is covered by newly regenerated hairs. e, Plasma levels of corticosterone in young mice (P46, P77, and P98) and aged mice (P427 and P581). f, Sham and ADX operations were performed on aged mice (P521). The mice were shaved and monitored for hair coat recovery from P521 to P574. Scale bars (b, c), 50 μm. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ****P < 0.0001. For exact P values, see Source Data. For statistics, sample sizes and numbers of replications, see Methods.
Extended Data Fig. 4 GR depletion in different cell types in the skin.
a, Top, K15-CrePGR depletes GR efficiently in HFSCs. Bottom, immunohistochemical analysis (GR and CD140a) of telogen hair follicle in the skin of control and K15-CrePGR;GRfl/fl mice. b, Hair cycle progression of control and K15-CrePGR;GRfl/fl mice. c, Immunohistochemical analyses (GR and PCAD) of telogen hair follicles in the skin of control and Pdgfra-CreER;GRfl/fl mice, showing that Pdgfra-CreER depletes GR efficiently in the dermal fibroblasts and DP. d, Immunocolocalization (EdU and CD34) in control and Pdgfra-CreER;GRfl/fl hair follicles after tamoxifen administration. EdU incorporation reveals premature HFSC activation in the hair follicles of Pdgfra-CreER;GRfl/fl mice. e, Comparison of EdU localization in bulge and upper ORS in late anagen (AnaV) of control (P124) and Pdgfra-CreER;GRfl/fl (P73) mice. f, Representative hair regeneration status of control and Pdgfra-CreER;GRfl/fl mice from P73 to P205, with quantification of the number of hair cycles. g, Immunocolocalization (EdU and CD34) in infundibulum, junctional zone, sebaceous gland, mid ORS, lower ORS, and matrix of late anagen (AnaVI) hair follicles in control and Pdgfra-CreER;GRfl/fl mice during late anagen with quantifications. h, Top, H&E staining in the late anagen skin of control and Pdgfra-CreER;GRfl/fl mice, with quantification of the thickness of epidermis (E). Bottom, immunocolocalization (EdU and DAPI) in interfollicular epidermis and dermis in control and Pdgfra-CreER;GRfl/fl mice. Scale bars (a, c–e, g, h), 50 μm. Yellow dashed lines, bulge (a, c–e); white dashed lines, hair germ (a, c, d), the rest of hair follicles (e, g), or the boundary between the epidermis and the dermis (h); solid white line, DP (a, c, d). Data are mean ± s.e.m. **P < 0.01, ***P < 0.001, ****P < 0.0001, NS, not significant. For exact P values, see Source Data. For statistics, sample sizes and numbers of replications, see Methods.
Extended Data Fig. 5 Corticosterone acts on the DP.
a, RT–qPCR of GR from DP and DF. b, Immunohistochemical analyses (YFP and DAPI) of anagen skin from Sox2-CreER;R26-lsl-YFP mice. Left, the arrowhead indicates an anagen guard hair follicle with YFP+ DP cells. Right, quantification of the percentage of YFP+ and YFP− DP in Sox2-CreER;R26-lsl-YFP. Only guard hair follicles have YFP+ DP. c, Immunohistochemical analyses (GR and DAPI) of skin from control and Sox2-CreER;GRfl/fl mice. Dashed lines, epidermis and hair follicles; solid line, DP. The arrowhead indicates the DP of Sox2-CreER;GRfl/fl guard hairs. d, Representative hair regeneration status of control and Sox2-CreER;GR fl/fl mice from P45 to P160. Quantification shows the number of hair cycles for guard hairs and other hairs in control and Sox2-CreER;GRfl/fl mice. e, Comparison of the hair bulb diameter in late anagen (AnaV) in the skin of control (P120) and Sox2-CreER;GRfl/fl (P67) mice. Yellow lines indicate the hair bulb diameter. The arrowhead denotes minor hyper thickening of the Sox2-CreER;GRfl/fl hair follicle around the ORS, probably because the dermis has not expanded to accommodate the extra proliferation from HFSCs. f, Immunocolocalization of phosphohistone H3 (pHH3) and CD34 in bulge and upper ORS, middle ORS, lower ORS and matrix of late anagen (AnaV) guard hair follicles in control and Sox2-CreER;GRfl/fl mice. The white arrowhead denotes a thickened region in a Sox2-CreER;GRfl/fl hair follicle, probably due to excessive proliferation from HFSCs. Yellow dashed lines, bulge; white dashed lines, the rest of the hair follicle. g, Hair shaft length of guard hairs in control and Sox2-CreER;GRfl/fl mice after anagen. Scale bars, 50 μm (b, c, e, f), 1 mm (d, g). Data are mean ± s.e.m. ****P < 0.0001, NS, not significant. For exact P values, see Source Data. For statistics, sample sizes and numbers of replications, see Methods.
Extended Data Fig. 6 Differential gene expression in HFSCs of control, ADX and dermal GR-knockout mice.
a, Sample clustering based on Pearson’s correlation of transcriptomes in HFSCs from sham, ADX, and ADX+CORT as well as control and Pdgfra-CreER;GRfl/fl mice. b, Principal component analysis (PCA) comparing the transcriptome of HFSCs from sham, ADX, ADX+CORT, control and Pdgfra-CreER;GRfl/fl mice. c, Heat map of log2-transformed fold change of gene expression of 121 common genes among ADX (versus sham), Pdgfra-CreER;GRfl/fl (versus control), and ADX+CORT (versus ADX). Cell-cycle-related genes are noted in orange. d, Left, heat map of log2-transformed fold change of gene expression of transcription factors (Foxc1, Lhx2, Foxp1, Nfatc1), key signalling factors (Fgf18), or downstream readout of key signalling factors (Id1 for BMP pathway, Axin2 for WNT pathway, Gli1 for SHH pathway) known to regulate HFSC quiescence. Right, heat map of log2-transformed fold change of gene expression of 7 core genes related to cell-cycle machineries and cytokinesis. e, f, RT–qPCR of genes related to cell-cycle machineries and cytokinesis from telogen HFSCs of sham and ADX mice (e) and control and Pdgfra-CreER;GRfl/fl mice (f). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. For exact P values, see Source Data. For statistics, sample sizes and numbers of replications, see Methods.
Extended Data Fig. 7 The expression of cell-cycle-related genes in HFSCs.
a, b, RT–qPCR of genes related to cell-cycle machineries and cytokinesis using telogen HFSCs from vehicle and corticosterone-fed mice (a) and control and stressed mice (b). c, d, RT–qPCR of genes related to cell-cycle machineries and cytokinesis in telogen epidermis from sham and ADX mice (c) and vehicle and corticosterone-fed mice (d). e, Experimental workflow of the differentially expressed genes (DEGs, >1.5-fold, Padj < 0.05) from DP cells of sham and ADX mice, as well as control and Pdgfra-CreER;GRfl/fl mice. f, g, Immunohistochemical analysis (PCAD) of skin samples from sham and ADX (f) or control and Pdgfra-CreER;GRfl/fl (g) mice used in RNA-seq experiments to validate hair cycle (all telogen). Dashed lines, epidermis and hair follicles. h, Top, FACS strategies for isolating DP cells for RNA-seq24,44. Bottom, the expression levels of cell-type-specific signature genes (DP, fibroblasts, HFSCs and mast cells) in FACS-purified DP cells. TPM, transcripts per million. i, Sample clustering based on Pearson’s correlation of transcriptomes in DP of sham and ADX mice (left), as well as control and Pdgfra-CreER;GRfl/fl mice (right). j, Heat maps of the differentially expressed genes (DEGs, > 1.5-fold, Padj < 0.05) from FACS-purified DP cells of sham and ADX mice (left) or control and Pdgfra-CreER;GRfl/fl mice (right). Scale bars (f, g), 50 μm. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, NS, not significant. For exact P values, see Source Data. For statistics, sample sizes and numbers of replications, see Methods.
Extended Data Fig. 8 Transcriptome analysis and secretome analysis identified GAS6 as a secreted factor suppressed by systemic corticosterone in the DP.
a, Secretome analysis identifying common secreted factors from DEGs (>1.5-fold, Padj < 0.05) in ADX and Pdgfra-CreER;GRfl/fl DP cells identified by RNA-seq. b, c, Expression levels of shared differentially expressed secreted factors as TPM, in the DP cells of ADX (b) and Pdgfra-CreER;GRfl/fl (c) mice. d, Top, negative control and Gas6 mRNA expression by in situ hybridization in late anagen (AnaV) and mid catagen (CatV) skin of sham and ADX mice. Bottom, quantification of Gas6 mRNA in the DP. Dashed lines, hair follicle; solid lines, DP. e, Representative image of negative control and Axl mRNA expression by in situ hybridization in telogen skin. Yellow dashed lines: bulge; white dashed lines: hair germ (top). RT–qPCR of Axl, Tgfbr1, Bmpr1a, Nfatc1 and PPIB from HFSCs and epidermal stem cells (EpSCs) of control mice (P83) (bottom). f, Representative images of negative control and Axl mRNA expression by in situ hybridization in late anagen skin. Yellow dashed lines: bulge; white dashed lines: epidermis and hair follicles. g, The expression levels (as TPM) of genes encoding TAM receptors (Tyro3, Axl and Mertk) in HFSCs. h, Left, schematic of the GAS6–AXL receptor tyrosine kinase pathway. R428 is a selective inhibitor of AXL tyrosine kinase activity43. Right, colony-formation assays of cultured HFSCs in R428 or GAS6 with R428 with quantifications. Scale bars (d–f), 50 μm. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS, not significant. For exact P values, see Source Data. For statistics, sample sizes and numbers of replications, see Methods.
Extended Data Fig. 9 Analyses of skin changes upon Gas6 overexpression or treatment with an AXL inhibitor.
a, Immunohistochemical analysis (GFP and PCAD) of PBS-injected second telogen skin and AAV-GFP-injected second telogen skin. Dashed lines, epidermis and hair follicles; solid lines: DP. b, RT–qPCR of Gas6 from dermal fibroblasts of PBS-injected second telogen skin (control) and AAV-CAG-Gas6-injected second telogen skin. c, Precocious HFSC activation in mice injected with AAV-CAG-Gas6 shown by EdU incorporation. Immunocolocalization (EdU and CD34) in control and AAV-CAG-Gas6-injected skin after AAV injection (D3 to D9). d, Comparison of EdU and CD34 localization in bulge and upper outer root sheath (ORS) in late anagen (AnaV) (control, D50 after injection; GAS6, D17 after injection). e, H&E staining of late anagen (AnaVI) skin (control, D53 after injection; GAS6, D20 after injection). Quantification of the ORS length in the zigzag hairs of control and AAV-CAG-Gas6-injected mice during late anagen. Brackets indicate the ORS length below the bulge. f, Immunocolocalization (EdU and CD34) in infundibulum, junctional zone, sebaceous gland, mid ORS, lower ORS and matrix of late anagen (AnaVI) hair follicles in control and AAV-CAG-Gas6-injected mice with quantifications. Dashed lines outline hair follicles. g, Top, H&E staining in the late anagen skin of control and AAV-CAG-Gas6-injected mice with quantification of the thickness of epidermis (E). Bottom, immunocolocalization (EdU and DAPI) in interfollicular epidermis and dermis in control and AAV-CAG-Gas6-injected mice with quantifications. h, RT–qPCR of genes related to HFSC proliferation in HFSCs of second telogen skin. i, Hair cycle progression of sham and ADX mice treated with ethanol topically, or ADX mice treated with R428 in ethanol. j, RT–qPCR of genes related to cell-cycle machineries and cytokinesis from HFSCs of sham and ADX mice treated with ethanol topically, or ADX mice treated for 7 days with R428 (in ethanol) topically. Yellow dashed lines, bulge (c, d); white dashed lines, hair germ (c), the rest of the hair follicle (d, f), or the boundary of epidermis and dermis (g); solid white line, DP (c). EtOH, ethanol. Scale bars (a, c–g), 50 μm. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS, not significant. For exact P values, see Source Data. For statistics, sample sizes and numbers of replications, see Methods.
Extended Data Fig. 10 Interactions between systemic corticosterone and local BMP signalling.
a, In situ hybridization of negative control and Gas6 mRNA expression in different conditions, including P49 (early telogen) of sham mice, P80 (late telogen) of sham mice, P49 of ADX mice, P49 of in sham mice injected with AAV-CAG-Noggin (Noggin in sham), and P49 in ADX mice after injection of AAV-CAG-Noggin (Noggin in ADX). Quantifications show in situ hybridization signal intensities in the DP. The model shows how changes in corticosterone and BMP signalling influence Gas6 levels in the DP. b, RT–qPCR of Gas6 from the DP of P49 (early telogen) and P80 (late telogen) of sham mice, and P49 of ADX mice, Noggin in sham mice, and Noggin in ADX mice. c, Hair cycle progression in sham, ADX, Noggin in sham, and Noggin in ADX mice with quantifications. Dashed circles indicate AAV-CAG-Noggin injection areas. d, Control and Pdgfra-CreER;GRfl/fl mice were subjected to chronic unpredictable stress from P55. Quantification shows the percentage of hair regrowth at P107. e, RT–qPCR of Gas6 from DP cells of control (P83) and stressed (P83) mice (left), and vehicle (P83) and corticosterone-fed (P83) mice (right). f, In situ hybridization of Gas6 in vehicle (P83, late telogen) and corticosterone-fed (P83) mice, with quantification of in situ signals in DP. g, Model of corticosterone regulation of telogen length. In normal conditions, corticosterone levels remain constant, but BMP levels naturally decrease as telogen progresses, until a point is reached at which Gas6 levels are sufficiently increased so as to drive HFSCs out of quiescence. This dynamic can be altered by changing either the corticosterone level or the BMP level. If corticosterone levels decrease, the sum of inhibitory cues on Gas6 falls below a critical threshold sooner in telogen, leading to increased Gas6 levels and precocious anagen entry. In the case of stress, age, or corticosterone feeding, increased corticosterone levels reduce Gas6 levels to below the critical threshold, leading to an extended telogen. Dashed lines, hair follicles; solid lines, DP. Scale bars (a, f), 50 μm. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS, not significant. For exact P values, see Source Data. For statistics, sample sizes and numbers of replications, see Methods.
Supplementary information
Supplementary Information
This file contains the Supplementary Discussion, Supplementary References and Supplementary Figure 1.
Source data
Rights and permissions
About this article
Cite this article
Choi, S., Zhang, B., Ma, S. et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 592, 428–432 (2021). https://doi.org/10.1038/s41586-021-03417-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03417-2
This article is cited by
-
Autophagy induces hair follicle stem cell activation and hair follicle regeneration by regulating glycolysis
Cell & Bioscience (2024)
-
FOXO1-mediated lipid metabolism maintains mammalian embryos in dormancy
Nature Cell Biology (2024)
-
Deciphering the molecular mechanisms of stem cell dynamics in hair follicle regeneration
Experimental & Molecular Medicine (2024)
-
Local and systemic mechanisms that control the hair follicle stem cell niche
Nature Reviews Molecular Cell Biology (2024)
-
The journey from melanocytes to melanoma
Nature Reviews Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.