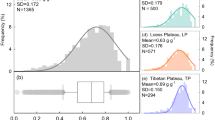
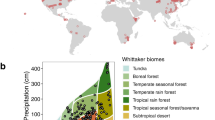
Abstract
One of the most notable ecological trends—described more than 2,300 years ago by Theophrastus—is the association of small leaves with dry and cold climates, which has recently been recognized for eudicotyledonous plants at a global scale1,2,3. For eudicotyledons, this pattern has been attributed to the fact that small leaves have a thinner boundary layer that helps to avoid extreme leaf temperatures4 and their leaf development results in vein traits that improve water transport under cold or dry climates5,6. However, the global distribution of leaf size and its adaptive basis have not been tested in the grasses, which represent a diverse lineage that is distinct in leaf morphology and that contributes 33% of terrestrial primary productivity (including the bulk of crop production)7. Here we demonstrate that grasses have shorter and narrower leaves under colder and drier climates worldwide. We show that small grass leaves have thermal advantages and vein development that contrast with those of eudicotyledons, but that also explain the abundance of small leaves in cold and dry climates. The worldwide distribution of leaf size in grasses exemplifies how biophysical and developmental processes result in convergence across major lineages in adaptation to climate globally, and highlights the importance of leaf size and venation architecture for grass performance in past, present and future ecosystems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data are available in the Article and its Supplementary Information. Leaf trait data for the 1,752 grass species was provided by the published Kew Grassbase Database (http://www.kew.org/data/grassbase/). Climate data for species were extracted from WorldClim 2 5-arc minute resolution (https://www.worldclim.org/) and from CRU TS4.01 01 (https://crudata.uea.ac.uk/cru/data/hrg/cru_ts_4.01/) on the basis of the geographical records for each species (http://www.gbif.org). Photosynthetic trait data and field locations were extracted for the 13 C3 grass species for which this was available in GLOPNET (http://bio.mq.edu.au/~iwright/glopian.htm). Source data are provided with this paper.
Code availability
Custom-written R code is available on GitHub (https://github.com/smuel-tylor/grass-leaf-size-).
References
Hort, A. Enquiry into Plants, Vol. I, by Theophrastus (Harvard Univ. Press, 1948).
Peppe, D. J. et al. Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytol. 190, 724–739 (2011).
Wright, I. J. et al. Global climatic drivers of leaf size. Science 357, 917–921 (2017).
Gates, D. M. Transpiration and leaf temperature. Annu. Rev. Plant Physiol. 19, 211–238 (1968).
Sack, L. et al. Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat. Commun. 3, 837 (2012).
Sack, L. & Scoffoni, C. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 198, 983–1000 (2013).
Beer, C. et al. Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329, 834–838 (2010).
Gallaher, T. J. et al. Leaf shape and size track habitat transitions across forest-grassland boundaries in the grass family (Poaceae). Evolution 73, 927–946 (2019).
Soreng, R. J. et al. A worldwide phylogenetic classification of the Poaceae (Gramineae) II: an update and a comparison of two 2015 classifications. J. Syst. Evol. 55, 259–290 (2017).
Schuepp, P. H. Tansley review no. 59 leaf boundary layers. New Phytol. 125, 477–507 (1993).
Orians, G. H. & Solbrig, O. T. A cost–income model of leaves and roots with special reference to arid and semiarid areas. Am. Nat. 111, 677–690 (1977).
Körner, C. Plant adaptation to cold climates. F1000Res. 5, 2769 (2016).
Niklas, K. J. Plant Allometry: The Scaling of Form and Process (Univ. Chicago Press, 1994).
Nelson, T. & Dengler, N. Leaf vascular pattern formation. Plant Cell 9, 1121–1135 (1997).
Christin, P. A. et al. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc. Natl Acad. Sci. USA 110, 1381–1386 (2013).
Ueno, O., Kawano, Y., Wakayama, M. & Takeda, T. Leaf vascular systems in C3 and C4 grasses: a two-dimensional analysis. Ann. Bot. 97, 611–621 (2006).
Sage, R. F. The evolution of C4 photosynthesis. New Phytol. 161, 341–370 (2004).
Clayton, W. D., Vorontsova, M. S., Harman, K. T. & Williamson, H. GrassBase–The Online World Grass Flora http://www.kew.org/data/grasses-db.html (2006).
Parkhurst, D. F. & Loucks, O. L. Optimal leaf size in relation to environment. J. Ecol. 60, 505–537 (1972).
Okajima, Y., Taneda, H., Noguchi, K. & Terashima, I. Optimum leaf size predicted by a novel leaf energy balance model incorporating dependencies of photosynthesis on light and temperature. Ecol. Res. 27, 333–346 (2012).
Davis, S. D., Sperry, J. S. & Hacke, U. G. The relationship between xylem conduit diameter and cavitation caused by freezing. Am. J. Bot. 86, 1367–1372 (1999).
Blackman, C. J., Brodribb, T. J. & Jordan, G. J. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol. 188, 1113–1123 (2010).
Scoffoni, C., Rawls, M., McKown, A., Cochard, H. & Sack, L. Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiol. 156, 832–843 (2011).
Scoffoni, C. et al. Leaf vein xylem conduit diameter influences susceptibility to embolism and hydraulic decline. New Phytol. 213, 1076–1092 (2017).
Craine, J. M. et al. Global diversity of drought tolerance and grassland climate-change resilience. Nat. Clim. Chang. 3, 63–67 (2013).
Scoffoni, C. et al. Hydraulic basis for the evolution of photosynthetic productivity. Nat. Plants 2, 16072 (2016).
Jones, H. G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology 3rd edn (Cambridge Univ. Press, 2014).
Grace, J. Plant–Atmosphere Relationships 1st edn (Chapman and Hall, 1983).
Weiser, R. L., Asrar, G., Miller, G. P. & Kanemasu, E. T. Assessing grassland biophysical characteristics from spectral measurements. Remote Sens. Environ. 20, 141–152 (1986).
Meinzer, F. C. & Grantz, D. A. Stomatal control of transpiration from a developing sugarcane canopy. Plant Cell Environ. 12, 635–642 (1989).
Liu, H. et al. Life history is a key factor explaining functional trait diversity among subtropical grasses, and its influence differs between C3 and C4 species. J. Exp. Bot. 70, 1567–1580 (2019).
Fort, F., Jouany, C. & Cruz, P. Root and leaf functional trait relations in Poaceae species: Implications of differing resource-acquisition strategies. J. Plant Ecol. 6, 211–219 (2013).
Holloway-Phillips, M. M. & Brodribb, T. J. Contrasting hydraulic regulation in closely related forage grasses: implications for plant water use. Funct. Plant Biol. 38, 594–605 (2011).
Brodribb, T. J., Feild, T. S. & Sack, L. Viewing leaf structure and evolution from a hydraulic perspective. Funct. Plant Biol. 37, 488–498 (2010).
Linacre, E. T. Further notes on a feature of leaf and air temperatures. Archiv Meteorol. Geophys. Bioklimatol. B 15, 422–436 (1967).
John, G. P. et al. The anatomical and compositional basis of leaf mass per area. Ecol. Lett. 20, 412–425 (2017).
Givnish, T. J. Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol. 106, 131–160 (1987).
Lusk, C. H., Grierson, E. R. P. & Laughlin, D. C. Large leaves in warm, moist environments confer an advantage in seedling light interception efficiency. New Phytol. 223, 1319–1327 (2019).
Olson, M. E. et al. Plant height and hydraulic vulnerability to drought and cold. Proc. Natl Acad. Sci. USA 115, 7551–7556 (2018).
Niklas, K. J. A mechanical perspective on foliage leaf form and function. New Phytol. 143, 19–31 (1999).
Merkhofer, L. et al. Resolving Australian analogs for an Eocene Patagonian paleorainforest using leaf size and floristics. Am. J. Bot. 102, 1160–1173 (2015).
Somerville, C. The billion-ton biofuels vision. Science 312, 1277 (2006).
Sedelnikova, O. V., Hughes, T. E. & Langdale, J. A. Understanding the genetic basis of C4 kranz anatomy with a view to engineering C3 crops. Annu. Rev. Genet. 52, 249–270 (2018).
Sage, R. F. & Zhu, X. G. Exploiting the engine of C4 photosynthesis. J. Exp. Bot. 62, 2989–3000 (2011).
Feldman, A. B. et al. Increasing leaf vein density via mutagenesis in rice results in an enhanced rate of photosynthesis, smaller cell sizes and can reduce interveinal mesophyll cell number. Front. Plant Sci. 8, 1883 (2017).
Edwards, E. J. et al. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328, 587–591 (2010).
Linder, H. P., Lehmann, C. E. R., Archibald, S., Osborne, C. P. & Richardson, D. M. Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biol. Rev. Camb. Philos. Soc. 93, 1125–1144 (2018).
Kluyver, T. A. & Osborne, C. P. Taxonome: a software package for linking biological species data. Ecol. Evol. 3, 1262–1265 (2013).
Cayuela, L., Granzow-de la Cerda, I., Albuquerque, F. S. & Golicher, D. J. TAXONSTAND: an R package for species names standardisation in vegetation databases. Methods Ecol. Evol. 3, 1078–1083 (2012).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Cherlet, M. H. C., Reynolds, J., Hill, J., Sommer, S. & von Maltitz, G. World Atlas of Desertification 3rd edn (Publication Office of the European Union, 2018).
Harris, I., Jones, P. D., Osborn, T. J. & Lister, D. H. Updated high-resolution grids of monthly climatic observations – the CRU TS3.10 dataset. Int. J. Climatol. 34, 623–642 (2014).
Lasky, J. R. et al. Characterizing genomic variation of Arabidopsis thaliana: the roles of geography and climate. Mol. Ecol. 21, 5512–5529 (2012).
Sexton, J. P., McIntyre, P. J., Angert, A. L. & Rice, K. J. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 40, 415–436 (2009).
Dengler, N. G., Dengler, R. E. & Hattersley, P. W. Differing ontogenetic origins of PCR (Kranz) sheaths in leaf blades of C4 grasses (Poaceae). Am. J. Bot. 72, 284–302 (1985).
Dengler, N. G., Woodvine, M. A., Donnelly, P. M. & Dengler, R. E. Formation of vascular pattern in developing leaves of the C4 grass Arundinella hirta. Int. J. Plant Sci. 158, 1–12 (1997).
Ikenberry, G.-J. J. Developmental Vegetative Morphology of Avena sativa. PhD thesis, Iowa State Univ. (1959).
Kaufman, P. B. & Brock, T. G. in Oat Science and Technology (eds Marshall, H. G. & Sorrells, M. E.) 53–75 (American Society of Agronomy, 1992).
Hitch, P. A. & Sharman, B. C. Initiation of procambial strands in leaf primordia of Dactylis glomerata L as an example of a temperate herbage grass. Ann. Bot. 32, 153–164 (1968).
Davidson, J. L. & Milthorpe, F. L. Leaf growth in Dactylis glomerata following defoliation. Ann. Bot. 30, 173–184 (1966).
Volenec, J. J. & Nelson, C. J. Cell dynamics in leaf meristems of contrasting tall fescue genotypes. Crop Sci. 21, 381–385 (1981).
Macadam, J. W. & Nelson, C. J. Specific leaf weight in zones of cell division, elongation and maturation in tall fescue leaf blades. Ann. Bot. 59, 369–376 (1987).
MacAdam, J. W., Volenec, J. J. & Nelson, C. J. Effects of nitrogen on mesophyll cell division and epidermal cell elongation in tall fescue leaf blades. Plant Physiol. 89, 549–556 (1989).
Skinner, R. H. & Nelson, C. J. Elongation of the grass leaf and its relationship to the phyllochron. Crop Sci. 35, 4–10 (1995).
Skinner, R. H. & Nelson, C. J. Epidermal cell division and the coordination of leaf and tiller development. Ann. Bot. 74, 9–15 (1994).
Maurice, I., Gastal, F. & Durand, J. L. Generation of form and associated mass deposition during leaf development in grasses: a kinematic approach for non-steady growth. Ann. Bot. 80, 673–683 (1997).
Durand, J. L., Schaufele, R. & Gastal, F. Grass leaf elongation rate as a function of developmental stage and temperature: morphological analysis and modelling. Ann. Bot. 83, 577–588 (1999).
Martre, P., Durand, J. L. & Cochard, H. Changes in axial hydraulic conductivity along elongating leaf blades in relation to xylem maturation in tall fescue. New Phytol. 146, 235–247 (2000).
Martre, P. & Durand, J. L. Quantitative analysis of vasculature in the leaves of Festuca arundinacea (Poaceae): Implications for axial water transport. Int. J. Plant Sci. 162, 755–766 (2001).
Gallagher, J. N. Field studies of cereal leaf growth 1. Initiation and expansion in relation to temperature and ontogeny. J. Exp. Bot. 30, 625–636 (1979).
Gallagher, J. N. & Biscoe, P. V. Field studies of cereal leaf growth 3. Barley leaf extension in relation to temperature, orradiance, and water potential. J. Exp. Bot. 30, 645–655 (1979).
Dannenhoffer, J. M., Ebert, W. & Evert, R. F. Leaf vasculature in barley, Hordeum vulgare (Poaceae). Am. J. Bot. 77, 636–652 (1990).
Dannenhoffer, J. M. & Evert, R. F. Development of the vascular system in the leaf of barley (Hordeum vulgare L). Int. J. Plant Sci. 155, 143–157 (1994).
Trivett, C. L. & Evert, R. F. Ontogeny of the vascular bundles and contiguous tissues in the barley leaf blade. Int. J. Plant Sci. 159, 716–723 (1998).
Soper, K. & Mitchell, K. J. The developmental anatomy of perennial ryegrass (Lolium perenne L.). N. Z. J. Sci. Technol. 37, 484–504 (1956).
Schnyder, H., Nelson, C. J. & Coutts, J. H. Assessment of spatial distribution of growth in the elongation zone of grass leaf blades. Plant Physiol. 85, 290–293 (1987).
Arredondo, J. T. & Schnyder, H. Components of leaf elongation rate and their relationship to specific leaf area in contrasting grasses. New Phytol. 158, 305–314 (2003).
Kaufman, P. B. Development of the shoot of Oryza sativa L. – II. Leaf histogenesis. Phytomorphology 9, 277–311 (1959).
Yamazaki, K. Studies on the leaf formation in rice plants: I. Observation on the successive development of the leaf. Jpn. J. Crop. Sci. 31, 371–378 (1963).
Chonan, N. K. H. & Matsuda, T. Morphology on vascular bundles of leaves in gramineous crops: I. Observations on vascular bundles of leaf blades, sheaths and internodes in riceplants. Jpn. J. Crop. Sci. 43, 425–432 (1974).
Hoshikawa, K. The Growing Rice Plant: An Anatomical Monograph (Nobunkyo, 1989).
Matsukura, C. et al. Transverse vein differentiation associated with gas space formation – fate of the middle cell layer in leaf sheath development of rice. Ann. Bot. 85, 19–27 (2000).
Itoh, J. et al. Rice plant development: from zygote to spikelet. Plant Cell Physiol. 46, 23–47 (2005).
Sakaguchi, J. & Fukuda, H. Cell differentiation in the longitudinal veins and formation of commissural veins in rice (Oryza sativa) and maize (Zea mays). J. Plant Res. 121, 593–602 (2008).
Parent, B., Conejero, G. & Tardieu, F. Spatial and temporal analysis of non-steady elongation of rice leaves. Plant Cell Environ. 32, 1561–1572 (2009).
Begg, J. E. & Wright, M. J. Growth and development of leaves from intercalary meristems in Phalaris arundinacea L. Nature 194, 1097–1098 (1962).
Colbert, J. T. & Evert, R. F. Leaf vasculature in sugarcane (Saccharum officinarum L.). Planta 156, 136–151 (1982).
Bernstein, N., Silk, W. K. & Lauchli, A. Growth and development of sorghum leaves under conditions of NaCl stress – spatial and temporal aspects of leaf growth inhibition. Planta 191, 433–439 (1993).
Sud, R. M. & Dengler, N. G. Cell lineage of vein formation in variegated leaves of the C4 grass Stenotaphrum secundatum. Ann. Bot. 86, 99–112 (2000).
Sharman, B. C. & Hitch, P. A. Initiation of procambial strands in leaf primordia of bread wheat Triticum aestivum L. Ann. Bot. 31, 229–243 (1967).
Blackman, E. The morphology and development of cross veins in the leaves of bread wheat (Triticum aestivum L.). Ann. Bot. 35, 653–665 (1971).
Kemp, D. R. The location and size of the extension zone of emerging wheat leaves. New Phytol. 84, 729–737 (1980).
Paolillo, D. J. Protoxylem maturation in the seedling leaf of wheat. Am. J. Bot. 82, 337–345 (1995).
Beemster, G. T. S. & Masle, J. The role of apical development around the time of leaf initiation in determining leaf width at maturity in wheat seedlings (Triticum aestivum L.) with impeded roots. J. Exp. Bot. 47, 1679–1688 (1996).
Sharman, B. C. Developmental anatomy of the shoot of Zea mays L. Ann. Bot. 6, 245–282 (1942).
Esau, K. Ontogeny of the vascular bundle in Zea mays. Hilgardia 15, 325–368 (1943).
Bosabalidis, A. M., Evert, R. F. & Russin, W. A. Ontogeny of the vascular bundles and contiguous tissues in the maize leaf blade. Am. J. Bot. 81, 745–752 (1994).
Poethig, S. in Contemporary Problems in Plant Anatomy (eds Dickison R. A. & White, W. C.) 235–259 (Academic, 1984).
Russell, S. H. & Evert, R. F. Leaf vasculature in Zea mays L. Planta 164, 448–458 (1985).
Smith, L. G., Greene, B., Veit, B. & Hake, S. A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development 116, 21–30 (1992).
Fournier, C. & Andrieu, B. A 3D architectural and process-based model of maize development. Ann. Bot. 81, 233–250 (1998).
Muller, B., Reymond, M. & Tardieu, F. The elongation rate at the base of a maize leaf shows an invariant pattern during both the steady-state elongation and the establishment of the elongation zone. J. Exp. Bot. 52, 1259–1268 (2001).
Muller, B. et al. Association of specific expansins with growth in maize leaves is maintained under environmental, genetic, and developmental sources of variation. Plant Physiol. 143, 278–290 (2007).
Johnston, R., Leiboff, S. & Scanlon, M. J. Ontogeny of the sheathing leaf base in maize (Zea mays). New Phytol. 205, 306–315 (2015).
Ben-Haj-Salah, H. & Tardieu, F. Temperature affects expansion rate of maize leaves without change in spatial distribution of cell length – analysis of the coordination between cell division and cell expansion. Plant Physiol. 109, 861–870 (1995).
Tardieu, F., Reymond, M., Hamard, P., Granier, C. & Muller, B. Spatial distributions of expansion rate, cell division rate and cell size in maize leaves: a synthesis of the effects of soil water status, evaporative demand and temperature. J. Exp. Bot. 51, 1505–1514 (2000).
Runions, A. et al. Modeling and visualization of leaf venation patterns. ACM Trans. Graphic. 24, 702–711 (2005).
Scarpella, E. & Meijer, A. H. Pattern formation in the vascular system of monocot and dicot plant species. New Phytol. 164, 209–242 (2004).
Baskin, T. I. Anisotropic expansion of the plant cell wall. Annu. Rev. Cell. Dev. 21, 203–222 (2005).
Fujita, H. & Mochizuki, A. The origin of the diversity of leaf venation pattern. Dev. Dyn. 235, 2710–2721 (2006).
Granier, C. & Tardieu, F. Multi-scale phenotyping of leaf expansion in response to environmental changes: the whole is more than the sum of parts. Plant Cell Environ. 32, 1175–1184 (2009).
Scarpella, E., Barkoulas, M. & Tsiantis, M. Control of leaf and vein development by auxin. Cold Spring Harb. Perspect. Biol. 2, a001511 (2010).
Gázquez, A. & Beemster, G. T. S. What determines organ size differences between species? A meta-analysis of the cellular basis. New Phytol. 215, 299–308 (2017).
Scarpella, E. The logic of plant vascular patterning. Polarity, continuity and plasticity in the formation of the veins and of their networks. Curr. Opin. Genet. Dev. 45, 34–43 (2017).
Berlyn, G. P. M. J. P. Botanical Microtechnique and Cytochemistry (Iowa State Univ. Press, 1976).
Kemp, C. D. Methods of estimating leaf area of grasses from linear measurements. Ann. Bot. 24, 491–499 (1960).
Stickler, F. C., Wearden, S. & Pauli, A. W. Leaf area determination in grain sorghum. Agronony 53, 187–188 (1961).
Shi, P. et al. Leaf area–length allometry and its implications in leaf shape evolution. Trees 33, 1073–1085 (2019).
Ellis, R. P. A procedure for standardizing comparative leaf anatomy in the Poaceae. I. The leaf blade as viewed in transverse section. Bothalia 12, 65–109 (1976).
Evert, R. F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development (John Wiley, 2006).
Neufeld, H. S. et al. Genotypic variability in vulnerability of leaf xylem to cavitation in water-stressed and well-irrigated sugarcane. Plant Physiol. 100, 1020–1028 (1992).
Tyree, M. T., Zimmermann, M. H. & Zimmermann, M. H. Xylem Structure and the Ascent of Sap 2nd edn (Springer, 2002).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Grass Phylogeny Working Group II. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol. 193, 304–312 (2012).
Taylor, S. H. et al. Photosynthetic pathway and ecological adaptation explain stomatal trait diversity amongst grasses. New Phytol. 193, 387–396 (2012).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Drummond, A. J. & Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (2007).
Christin, P. A. et al. Molecular dating, evolutionary rates, and the age of the grasses. Syst. Biol. 63, 153–165 (2014).
Prasad, V. et al. Late Cretaceous origin of the rice tribe provides evidence for early diversification in Poaceae. Nat. Commun. 2, 480 (2011).
R Core Team. R: A Language and Environment for Statistical Computing, http://www.R-project.org/ (R Foundation for Statistical Computing, 2019).
Paradis, E. & Schliep, K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2019).
Spriggs, E. L., Christin, P.-A. & Edwards, E. J. Data from: C4 photosynthesis promoted species diversification during the Miocene grassland expansion, https://doi.org/10.5061/dryad.74b5d (2015).
Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 125, 1–15 (1985).
Schmerler, S. B. et al. Evolution of leaf form correlates with tropical-temperate transitions in Viburnum (Adoxaceae). Proc. R. Soc. B. 279, 3905–3913 (2012).
Fletcher, L. R. et al. Evolution of leaf structure and drought tolerance in species of Californian Ceanothus. Am. J. Bot. 105, 1672–1687 (2018).
Bramer, I. et al. in Next Generation Biomonitoring: Part 1 (Advances in Ecological Research, volume 58) (eds. Bohan, D. A. et al.) 101–161 (Academic, 2018).
Zanne, A. E. et al. Three keys to the radiation of angiosperms into freezing environments. Nature 506, 89–92 (2014).
Watcharamongkol, T., Christin, P. A. & Osborne, C. P. C. C4 photosynthesis evolved in warm climates but promoted migration to cooler ones. Ecol. Lett. 21, 376–383 (2018).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference 2nd edn (Springer, 2002).
Gelman, A. & Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models (Cambridge Univ. Press, 2006).
Faraway, J. J. Linear Models with R (Chapman & Hall, 2009).
Murray, K. & Conner, M. M. Methods to quantify variable importance: implications for the analysis of noisy ecological data. Ecology 90, 348–355 (2009).
Westoby, M. & Wright, I. J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 21, 261–268 (2006).
Grubb, P. J. Trade-offs in interspecific comparisons in plant ecology and how plants overcome proposed constraints. Plant Ecol. Divers. 9, 3–33 (2016).
Cade, B. S. & Noon, B. R. A gentle introduction to quantile regression for ecologists. Front. Ecol. Environ. 1, 412–420 (2003).
Grubb, P. J., Coomes, D. A. & Metcalfe, D. J. Comment on “A brief history of seed size”. Science 310, 783 (2005).
Moles, A. T. et al. Global patterns in plant height. J. Ecol. 97, 923–932 (2009).
Pagel, M. Inferring the historical patterns of biological evolution. Nature 401, 877–884 (1999).
Freckleton, R. P., Harvey, P. H. & Pagel, M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 (2002).
Pinheiro, J. et al. nlme: linear and nonlinear mixed effect models. R package version 3.1-140, https://CRAN.R-project.org/package=nlme (2019).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Warton, D. I., Wright, I. J., Falster, D. S. & Westoby, M. Bivariate line-fitting methods for allometry. Biol. Rev. Camb. Philos. Soc. 81, 259–291 (2006).
Garland, T., Dickerman, A. W., Janis, C. M. & Jones, J. A. Phylogenetic analysis of covariance by computer-simulation. Syst. Biol. 42, 265–292 (1993).
Gross, J. & Ligges, U. nortest: tests for normality. R package version 1.0-4, https://cran.r-project.org/package=nortest (2015).
Poorter, H. & Sack, L. Pitfalls and possibilities in the analysis of biomass allocation patterns in plants. Front. Plant Sci. 3, 259 (2012).
Smith, R. J. Use and misuse of the reduced major axis for line-fitting. Am. J. Phys. Anthropol. 140, 476–486 (2009).
Gates, D. M. Energy, plants, and ecology. Ecology 46, 1–13 (1965).
Lusk, C. H. et al. Frost and leaf-size gradients in forests: global patterns and experimental evidence. New Phytol. 219, 565–573 (2018).
Muir, C. D. tealeaves: an R package for modelling leaf temperature using energy budgets. AoB Plants 11, plz054 (2019).
Taylor, S. H. et al. Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment. New Phytol. 185, 780–791 (2010).
Huang, M. et al. Air temperature optima of vegetation productivity across global biomes. Nat. Ecol. Evol. 3, 772–779 (2019).
Farquhar, G. D., von Caemmerer, S. & Berry, J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 (1980).
Bernacchi, C. J., Pimentel, C. & Long, S. P. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant Cell Environ. 26, 1419–1430 (2003).
Muir, C. D. Making pore choices: repeated regime shifts in stomatal ratio. Proc. R. Soc. B. 282, 1–9 (2012).
Brummitt, R. K. World Geographical Scheme for Recording Plant Distributions (Hunt Institute for Botanical Documentation, 2001).
Acknowledgements
We thank T. Cheng, W. Deng, A. C. Diener, A. Kooner, M. McMaster, C. Muir, S. Moshrefi, A. J. Patel, A. Sayari and M. S. Vorontsova for logistical assistance. Funding was provided by the US National Science Foundation (grants 1457279, 1951244 and 2017949), the Natural Environment Research Council (grants NE/DO13062/1 and NE/T000759/1) and a Royal Society University Research Fellowship (grant URF\R\180022).
Author information
Authors and Affiliations
Contributions
The project was conceptualized by A.S.B., S.H.T., C.P.O. and L.S. A.S.B., S.H.T., J.P.-K., C.V., Y.Z., T.W., C.S., E.J.E., P.-A.C., C.P.O. and L.S. performed data curation, and reviewed and edited the manuscript. A.S.B., S.H.T., J.P.-K., C.V., Y.Z., T.W., C.S., P.-A.C. and L.S. undertook formal analyses. C.P.O. and L.S. acquired funding. A.S.B., S.H.T., J.P.-K., T.W., C.S., E.J.E., P.-A.C., C.P.O. and L.S. performed the investigations. A.S.B., S.H.T., J.P.-K., T.W., C.S., E.J.E., P.-A.C., C.P.O. and L.S. developed the methodology. A.S.B., S.H.T., J.P.-K., C.P.O. and L.S. administered the project. A.S.B., S.H.T., J.P.-K., T.W., C.S., E.J.E., P.-A.C., C.P.O. and L.S. provided resources. A.S.B., S.H.T., T.W. and P.-A.C. wrote the software. A.S.B., S.H.T., J.P.-K., C.P.O. and L.S. supervised the project. A.S.B., S.H.T., C.P.O. and L.S. validated the data. A.S.B., S.H.T., T.W., C.V. and P.-A.C. provided the data visualization. A.S.B., S.H.T. and L.S. wrote the original draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Timothy Brodribb, Ian Wright and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Time-calibrated phylogenetic trees for 1,752 worldwide grass species and for 27 grass species grown in a greenhouse common garden.
a, Phylogeny for 1,752 species, trimmed from a previous publication132, used for analyses of global scaling of leaf size with climate. C3 and C4 species are in black and red, respectively (n = 840 and n = 912, respectively). b, Phylogeny for 27 species used for analyses of leaf vein scaling (black branches, 11 C3 grasses; red branches, 16 C4 grasses), emphasizing the inclusion of 11 independent C4 origins. c, d, Map of the distributions of the 11 C3 species (c) and 16 C4 species (d).
Extended Data Fig. 2 Worldwide relationships of grass leaf and plant dimensions with the native climate of species, the global distribution of grass leaf size, and the scaling of grass leaf and plant dimensions.
a–l, Relationship of leaf length (a–c), leaf width (d–f), leaf area (leaf width × leaf length) (g–i) and culm height (j–l) with MAT, MAP and the aridity index (AI). m–o, Average across species of leaf area for each country in the global database (International Working Group on Taxonomic Databases for Plant Sciences, TDWG level-3 spatial units165), including countries for which >20 species occur in the global database (21–547 species for each country; grey for countries with <20 species represented); that is, mean leaf area (m), median leaf area (n) and leaf area for the largest leafed species (o). p–u, The scaling of leaf area with leaf length (p) and leaf width (q), leaf area with culm height (r), culm height with leaf length (s) and leaf width (t), and leaf width with leaf length (u). Leaf trait and climate data are provided in Supplementary Table 2. n = 1,752 globally distributed grass species in a–i, p, q, u, and 1,729 in j–l, r–t. Corresponding regression coefficients for ahistorical analyses of relationships in a–l: 0.14, 0.17, 0.14, 0.26, 0.34, 0.28, 0.24, 0.31, 0.26, 0.24, 0.29 and 0.3. Two-tailed PRMA regressions were fitted for log(trait) = log(a) + b log(trait) in a–l, p–u. ***P < 0.001, **P < 0.01. P = 0.0099 (a), 7.8 × 10−9 (b), 4.2 × 10−9 (c), 0.004 (d), 1.8 × 10−8 (e), 2.4 × 10−11 (f), 0.0014 (g), 2.9 × 10−11 (h), 2.2 × 10−13 (i), 1.7 × 10−6 (j), 4.0 × 10−7 (k), 1.1 × 10−5 (l), about 0 (p), about 0 (q), 3.17 × 10−219 (r), 1.92 × 10−205 (s), 7.92 × 10−106 (t), 2.7 × 10−96 (u). C3 and C4 species are shown in red and blue, respectively.
Extended Data Fig. 3 Worldwide association of grass leaf size with the native climate of the species in 3D, and binned by 1/3rd lowest, middle and highest MAT or MAP in 2D.
a–d, Leaf area versus climate variables (that is, x = MAT and y = MAP) (a, c); horizontal axes are flipped (that is, leaf area versus x = MAP and y = MAT) in b, d. e–p, Relationship of leaf length (e–g), leaf width (h–j), leaf area (k–m) and culm height (n–p) to MAP. n = 584 globally distributed grass species in e–m, and 576 in n–p. q–z, aa, bb, Relationships of leaf length (q–s), leaf width (t–v), leaf area (w–y) and culm height (z, aa, bb) with MAT. n = 584 globally distributed grass species in e–m, q–y, and 576 for n–p, z, aa, bb. In a, b, data for all species in the global database (n = 1,752) are presented; in c, d, 29 species with MAT <0 °C are excluded, for a clearer view of the bulk of the species. Projected grey shadows in a–d represent the bivariate relationships. Parameters from multiple regression analysis are presented in Supplementary Table 8. Two-tailed ordinary least square regressions were fitted for log(trait) = log (a) + b log(climate variable) in e–z, aa, bb. ***P < 0.001, **P < 0.01. P = 8.1 × 10−5 (e), 2.2 × 10–5 (f), 0.0002 (g), 0.0094 (h), 8.4 × 10−28 (i), 1.7 × 10–18 (j), 0.0002 (k), 1.1 × 10−20 (l), 1.8 × 10−15 (m), 0.0028 (n), 4.7 × 10–22 (o), 2.2 × 10−10 (p), 0.0106 (q), 2.9 × 10−6 (r), 7.0 × 10−5 (t), 6.7 × 10−6 (u), 1.5 × 10−17 (v), 0.0001 (w), 7.9 × 10−8 (x), 2.6 × 10−11 (y), 1.3 × 10−5 (z), 1.7 × 10−9 (aa), 8.5 × 10−10 (bb). C3 and C4 species are shown in red and blue, respectively.
Extended Data Fig. 4 Quantile regression analyses of worldwide associations of grass leaf traits with the native climate of species.
a–l, Relationship of leaf length (a–c), leaf width (d–f), leaf area (g–i) and culm height (j–l) with MAT, MAP and the aridity index. n = 1,752 globally distributed grass species in a–i, and 1,729 in j–l. Two-tailed ordinary least square (solid lines) and 95% and 5% quantile regressions (dotted lines) were fitted for log(trait) = log(a) + b log(climate variable); quantile lines are drawn if significantly different in slope at P < 0.05. C3 and C4 species are in red and blue, respectively.
Extended Data Fig. 5 Worldwide associations of grass leaf and plant dimensions with the native climate of species for species with leaf width <8.16 cm or <4.47 cm (below the modelled threshold for damage owing to night-time chilling or overheating) and modelled leaf temperature difference from air temperature for amiphistomatous grass leaves under different air temperatures.
a–h, Relationship of leaf length (a, b), leaf width (c, d), leaf area (e, f) and culm height (g, h) to MAT and MAP for species with leaf width <8.16 cm. i–p, Relationships of leaf length (i, j), leaf width (k, l), leaf area (m, n) and culm height (o, p) to MAT and MAP for species with leaf width <4.47 cm. n = 1,748 globally distributed grass species for a–f, 1,725 for g, h, 1,716 for i–n and 1,694 for o, p. q–z, aa, bb, Simulations were run with stomatal conductance (mol m−2 s−1) 0.1 (q–t), 0.2 (u–x) and 0.4 (y, z, aa, bb), and wind speed (m s−1), at 0.1 (q, u, y), 0.5 (r, v, z), 1 (s, w, aa) and 2 (t, x, bb), with leaf width (cm) of 0.04, 0.1, 0.5, 0.9, 1.5, 2.7 and 11 shown as increasing darker blue lines. No difference in leaf temperature from air temperature line in red. Two-tailed ordinary least square regressions were fitted for log(trait) = log(a) + b log(climate variable) in a–p. ***P < 0.001, **P < 0.01, *P < 0.05. P = 2.1 × 10−8 (a), 6.2 × 10−13 (b), 4.7 × 10−29 (c), 6.2 × 10−48 (d), 2.0 × 10−24 (e), 6.8 × 10−40 (f), 1.9 × 10−24 (g), 1.3 × 10−33 (h), 2.4 × 10−7 (i), 7.4 × 10−11 (j), 1.0 × 10−26 (k), 3.4 × 10−39 (l), 5.4 × 10−22 (m), 9.8 × 10−33 (n), 4.4 × 10−22 (o), 3.8 × 10−29 (p). C3 and C4 species are shown in red and blue, respectively.
Extended Data Fig. 6 Worldwide scaling of grass VLA and vein diameter with leaf size and aridity of the native climate of the species, and of vein xylem conduit diameter with vein diameter.
a–d, Relationship of major VLA to leaf width (a, c), leaf area (b, d) and the aridity index (e) (in which lower values correspond to greater climatic aridity). f–q, Relationship of vein diameters to leaf length (f, i, l, o), leaf width (g, j, m, p) and leaf area (h, k, n, q). r–z, aa, bb, cc, Relationship of VLA to leaf length (r, u, x, aa), leaf width (s, v, y, bb) and leaf area (t, w, z, cc). dd, ee, ff, gg, Relationships of vein xylem conduit diameters with vein diameter of first-order veins (dd), second-order veins (ee), third-order veins (ff) and fourth-order veins (gg). n = 616 species in a, 600 in b, 170 in c, 166 in d, 21 in e, 27 in f–z, aa, bb, cc, dd, ee, ff and 7 in gg. Two-tailed ordinary least square regressions, PGLS or PRMA regressions were fitted for log(trait) = log(a) + b log(trait or climate variable) in a and b, c and d or e, respectively. PRMA or PGLS regressions were fitted for log(vein diameter or VLA) = log(a) + b log(leaf length, width or leaf area) in f–q and r–z, aa, bb, cc, respectively. PRMA regressions were fitted for log(xylem conduit diameter) = log(a) + b log(vein diameter) in dd, ee, ff, gg. *P < 0.05, **P < 0.01, ***P < 0.001. P = 9.4 × 10−250 (a), 1.6 × 10−139 (b), 7.0 × 10−46 (c), 1.0 × 10−31 (d), 0.0051 (e), 0.0007 (f), 3.0 × 10−5 (h), 3.9 × 10−6 (i), 0.0003 (k), 1.2 × 10−34 (s), 7.0 × 10−4 (t), 1.4 × 10−7 (v), 0.0167 (w), 0.0020 (bb), 0.0110 (dd) and 0.0004 (ee). Line parameters for f–z, aa, bb, cc are given in Table 1, Supplementary Table 10; line parameters for dd, ee, ff, gg are given in Supplementary Table 11. Significant relationships are plotted with PRMA to illustrate the central trends (Methods). C3 and C4 species are shown in white and grey, respectively. The s.e. for species trait values are given in Supplementary Table 3.
Extended Data Fig. 7 Scaling of leaf vein projected area, vein surface area and vein volume of given vein orders with leaf dimensions across 27 grass species grown experimentally.
a–l, Relationship of VPA to leaf length (a, d, g, j), leaf width (b, e, h, k) and leaf area (c, f, i, l). m–x, Relationship of VSA to leaf length (m, p, s, v), leaf width (n, q, t, w) and leaf area (o, r, u, x). y, z, aa, bb, cc, dd, ee, ff, gg, ii, Relationship of VVA to leaf length (y, bb, ee, hh), leaf width (z, cc, ff, ii) and leaf area (aa, dd, gg, jj). Two-tailed PGLS regressions were fitted for log(VPA, VSA or VVA) = log(a) + b log(leaf length, width or area) and drawn when significant. *P < 0.05, **P < 0.01, ***P < 0.001; line parameters are given in Supplementary Table 10. P = 0.0011 (a), 1.2 × 10−12 (b), 0.0011 (d), 7.0 × 10−5 (e), 0.0335 (g), 0.0161 (h), 0.0167 (k), 0.0011 (m), 1.2 × 10−12 (n), 0.0011 (p), 7.0 × 10−5 (q), 0.0335 (s), 0.0161 (t), 0.0167 (w), 8.2 × 10−6 (y), 5.4 × 10−6 (z), 5.2 × 10−5 (bb), 0.0037 (cc), 0.0093 (ff). Significant trends are plotted with PRMA to illustrate the central trends (Methods). The s.e. for species trait values are given in Supplementary Table 3. C3 and C4 species are in white and grey, respectively.
Extended Data Fig. 8 Partitioning of the contributions of given vein orders of the venation architecture of C3 and C4 grasses, with minor veins accounting for the differences in VLA.
a, Triticum aestivum, a C3 species. b, Aristida ternipes, a C4 species without fourth-order veins (C4–3L) (that is, third-order veins are the highest longitudinal vein order). c, Paspalum dilatum, a C4 species with fourth-order veins (C4–4L) (that is, fourth-order veins are the highest longitudinal vein order). d, VLA (cm per cm2) distribution across vein orders for each type (C3 n = 11, C4–3L = 9, C4–4L = 7). e–h, VLA (e), VSA (f), VPA (g) and VVA (h) distribution across vein orders for each type (C3, n = 11; C4, n = 16). Statistical comparisons by phylogenetic ANOVA are given in Supplementary Table 3.
Extended Data Fig. 9 Associations between light-saturated leaf photosynthetic rate and native climate and vein traits for terrestrial C3 species, and the scaling of VLA of transverse fifth-order veins with major VLA in 27 C3 and C4 grass species grown experimentally.
a–c, Relationship of area-based light-saturated photosynthetic rate (Aarea) measured with photosynthesis systems and MAT (a), MAP (b) and growing season length (GSL) (c). d–f, Relationship of light-saturated photosynthetic rate per area and VLAmajor (cm per cm2) (d) and major VSA (VSAmajor, unitless) (e), and transverse VLA (VLAtransverse) (cm per cm2) with VLAmajor. Points and lines in red represent eight terrestrial C3 grasses (from this study) grown in a greenhouse common garden related to the mean climate of their native distribution, supporting the assumption of a higher photosynthetic rate in colder and drier climates with shorter growing seasons. Open points represent 13 Northern Hemisphere temperate terrestrial C3 grass species from the global plant trait network (GLOPNET126) measured in the field, as related to the mean climate at their field site. Black lines represent the significant trend through all the points in a, c, which—given the disparate data sources combined here (and the consideration of field site rather than native range climate for the GLOPNET species) —provides strong support for the generality of the relationships of Aarea to MAT and growing season length. Notably, these are conservative tests of the relationships of photosynthetic rate with native climate, as measurements of Aarea that use the photosynthesis system chamber do not include the effect of the boundary layer conductance (which is made very high and invariant)27. Under natural conditions (and especially under slow wind speeds), smaller leaves would have a boundary layer conductance higher than that of larger leaves (as shown in the simulation in Extended Data Fig. 5), and thus—under natural conditions that included the effects of boundary layer—a stronger trend would be expected for small-leafed species in colder and drier climates to have higher photosynthetic rates than larger-leafed species of warm, moist climates. Two-tailed ordinary least square regressions or PRMA were fitted for log(trait) = log(a) + b log(trait or climate variable) in a–e and f, respectively. *P < 0.05, **P < 0.01, xP = 0.04 in a one-tailed test of the hypothesized positive correlation. P = 0.0301 (red line in a), 0.0071 (black line in a), 0.0183 (b), 0.0474 (red line in c), 0.0021 (black line in c), 0.0794 (d), 0.0138 (e), 0.0061 (f). Error bars represent s.e. in a–e. The s.e. for species trait values in f are given in Supplementary Table 3. C3 and C4 species are shown in white and grey, respectively, in e.
Extended Data Fig. 10 Estimating leaf size from venation traits that can be measured on small samples or fragments of grass leaves.
a, b, Leaf area (a) and leaf width (b) predicted from VLA of second-order veins. n = 600 and 616 species in a and b, respectively (Grassbase dataset, Supplementary Table 2). The relationships were fitted with two-tailed ordinary least square regressions. These relationships enable the determination of intact leaf size from fragments that include at least two second-order veins (including fragmentary fossil remains). The 95% confidence intervals are in blue and 95% prediction intervals in red. ***P < 0.001. P = 1.4 × 10−127 (a), 7.6 × 10−227 (b).
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-4, full descriptions for Supplementary Tables 1-12 (Supplementary Tables supplied separately) and Supplementary References.
Supplementary Tables
This file contains Supplementary Tables 1-12 – see Supplementary Information document for full descriptions.
Source data
Rights and permissions
About this article
Cite this article
Baird, A.S., Taylor, S.H., Pasquet-Kok, J. et al. Developmental and biophysical determinants of grass leaf size worldwide. Nature 592, 242–247 (2021). https://doi.org/10.1038/s41586-021-03370-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03370-0
This article is cited by
-
The scaling relationship between perianth fresh mass and area: proof of concept using Magnolia × soulangeana Soul.-Bod
Trees (2024)
-
Geographical variation in functional traits of leaves of Caryopteris mongholica and the role of climate
BMC Plant Biology (2023)
-
A generalized performance equation and its application in measuring the Gini index of leaf size inequality
Trees (2023)
-
Genetic basis of sorghum leaf width and its potential as a surrogate for transpiration efficiency
Theoretical and Applied Genetics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.