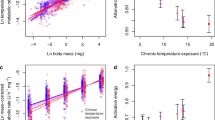
Abstract
In ecosystems, the efficiency of energy transfer from resources to consumers determines the biomass structure of food webs. As a general rule, about 10% of the energy produced in one trophic level makes it up to the next1,2,3. Recent theory suggests that this energy transfer could be further constrained if rising temperatures increase metabolic growth costs4, although experimental confirmation in whole ecosystems is lacking. Here we quantify nitrogen transfer efficiency—a proxy for overall energy transfer—in freshwater plankton in artificial ponds that have been exposed to seven years of experimental warming. We provide direct experimental evidence that, relative to ambient conditions, 4 °C of warming can decrease trophic transfer efficiency by up to 56%. In addition, the biomass of both phytoplankton and zooplankton was lower in the warmed ponds, which indicates major shifts in energy uptake, transformation and transfer5,6. These findings reconcile observed warming-driven changes in individual-level growth costs and in carbon-use efficiency across diverse taxa4,7,8,9,10 with increases in the ratio of total respiration to gross primary production at the ecosystem level11,12,13. Our results imply that an increasing proportion of the carbon fixed by photosynthesis will be lost to the atmosphere as the planet warms, impairing energy flux through food chains, which will have negative implications for larger consumers and for the functioning of entire ecosystems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data (data manipulation, analyses, figures and tables) can be downloaded from the GitHub repository https://github.com/dbarneche/nature20200508666. When using the data or code from this project, please cite https://doi.org/10.5281/zenodo.4468371.
Code availability
R code can be downloaded from the GitHub repository https://github.com/dbarneche/nature20200508666.
References
Semper, K. Animal Life as affected by the Natural Conditions of Existence (Appleton, 1881).
Lindeman, R. L. The trophic-dynamic aspect of ecology. Ecology 23, 399–417 (1942).
Pauly, D. & Christensen, V. Primary production required to sustain global fisheries. Nature 374, 255–257 (1995).
Barneche, D. R. & Allen, A. P. The energetics of fish growth and how it constrains food-web trophic structure. Ecol. Lett. 21, 836–844 (2018).
Allen, A. P., Gillooly, J. F. & Brown, J. H. Linking the global carbon cycle to individual metabolism. Funct. Ecol. 19, 202–213 (2005).
Yvon-Durocher, G. et al. Reconciling the temperature dependence of respiration across timescales and ecosystem types. Nature 487, 472–476 (2012).
Schaum, C. E., Buckling, A., Smirnoff, N., Studholme, D. J. & Yvon-Durocher, G. Environmental fluctuations accelerate molecular evolution of thermal tolerance in a marine diatom. Nat. Commun. 9, 1719 (2018).
Manzoni, S., Taylor, P., Richter, A., Porporato, A. & Ågren, G. I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 196, 79–91 (2012).
Bradford, M. A. & Crowther, T. W. Carbon use efficiency and storage in terrestrial ecosystems. New Phytol. 199, 7–9 (2013).
Barneche, D. R., Jahn, M. & Seebacher, F. Warming increases the cost of growth in a model vertebrate. Funct. Ecol. 33, 1256–1266 (2019).
Laws, E. A., Falkowski, P. G., Smith, W. O. Jr, Ducklow, H. & McCarthy, J. J. Temperature effects on export production in the open ocean. Glob. Biogeochem. Cycles 14, 1231–1246 (2000).
Davidson, E. A. & Janssens, I. A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173 (2006).
Yvon-Durocher, G., Hulatt, C. J., Woodward, G. & Trimmer, M. Long-term warming amplifies shifts in the carbon cycle of experimental ponds. Nat. Clim. Change 7, 209–213 (2017).
Elton, C. S. Animal Ecology (Macmillan, 1927).
Sheldon, R. W., Sutcliffe, W. H. Jr & Paranjape, M. A. Structure of pelagic food chain and relationship between plankton and fish production. J. Fish. Res. Board Can. 34, 2344–2353 (1977).
Brown, J. H. & Gillooly, J. F. Ecological food webs: high-quality data facilitate theoretical unification. Proc. Natl Acad. Sci. USA 100, 1467–1468 (2003).
Yvon-Durocher, G., Montoya, J. M., Trimmer, M. & Woodward, G. Warming alters the size spectrum and shifts the distribution of biomass in freshwater ecosystems. Glob. Change Biol. 17, 1681–1694 (2011).
Gasol, J. M., del Giorgio, P. A. & Duarte, C. M. Biomass distribution in marine planktonic communities. Limnol. Oceanogr. 42, 1353–1363 (1997).
Wang, H., Morrison, W., Singh, A. & Weiss, H. Modeling inverted biomass pyramids and refuges in ecosystems. Ecol. Modell. 220, 1376–1382 (2009).
Hutchinson, G. E. Homage to Santa Rosalia or why are there so many kinds of animals? Am. Nat. 93, 145–159 (1959).
Post, W. M. & Pimm, S. L. Community assembly and food web stability. Math. Biosci. 64, 169–192 (1983).
Šolić, M. et al. Impact of the 3 °C temperature rise on bacterial growth and carbon transfer towards higher trophic levels: Empirical models for the Adriatic Sea. J. Mar. Syst. 173, 81–89 (2017).
Ullah, H., Nagelkerken, I., Goldenberg, S. U. & Fordham, D. A. Climate change could drive marine food web collapse through altered trophic flows and cyanobacterial proliferation. PLoS Biol. 16, e2003446 (2018).
Jackson, J. B. C. et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637 (2001).
Stock, C. A. et al. Reconciling fisheries catch and ocean productivity. Proc. Natl Acad. Sci. USA 114, E1441–E1449 (2017).
Yvon-Durocher, G., Jones, J. I., Trimmer, M., Woodward, G. & Montoya, J. M. Warming alters the metabolic balance of ecosystems. Phil. Trans. R. Soc. B 365, 2117–2126 (2010).
Yvon-Durocher, G. et al. Five years of experimental warming increases the biodiversity and productivity of phytoplankton. PLoS Biol. 13, e1002324 (2015).
Mulholland, P. J. et al. Food resources of stream macroinvertebrates determined by natural-abundance stable C and N isotopes and a 15N tracer addition. J. North Am. Benthol. Soc. 19, 145–157 (2000).
Crossley, D. A. Jr & Reichle, D. E. Analysis of transient behavior of radioisotopes in insect food chains. Bioscience 19, 341–343 (1969).
Mulholland, P. J. et al. Stream denitrification and total nitrate uptake rates measured using a field 15N tracer addition approach. Limnol. Oceanogr. 49, 809–820 (2004).
López-Sepulcre, A. et al. A new method to reconstruct quantitative food webs and nutrient flows from isotope tracer addition experiments. Am. Nat. 195, 964–985 (2020).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M. & Charnov, E. L. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (2001).
Gillooly, J. F. et al. The metabolic basis of whole-organism RNA and phosphorus content. Proc. Natl Acad. Sci. USA 102, 11923–11927 (2005).
Allen, A. P. & Gillooly, J. F. Towards an integration of ecological stoichiometry and the metabolic theory of ecology to better understand nutrient cycling. Ecol. Lett. 12, 369–384 (2009).
Padfield, D., Buckling, A., Warfield, R., Lowe, C. & Yvon-Durocher, G. Linking phytoplankton community metabolism to the individual size distribution. Ecol. Lett. 21, 1152–1161 (2018).
Schaum, C. et al. Adaptation of phytoplankton to a decade of experimental warming linked to increased photosynthesis. Nat. Ecol. Evol. 1, 0094 (2017).
Rosenberg, A. A. et al. Developing new approaches to global stock status assessment and fishery production potential of the seas. Fisheries and Aquaculture Circular No. 1086 (FAO, 2014).
IPCC. Climate Change 2007: The Physical Science Basis (eds Solomon, S. et al.) (Cambridge Univ. Press, 2007).
Dossena, M. et al. Warming alters community size structure and ecosystem functioning. Proc. R. Soc. B 279, 3011–3019 (2012).
Lansdown, K. et al. Importance and controls of anaerobic ammonium oxidation influenced by riverbed geology. Nat. Geosci. 9, 357–360 (2016).
Stan Development Team. RStan: The R interface to Stan (2020).
Gelman, A. & Rubin, D. B. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472 (1992).
Bürkner, P. Advanced Bayesian multilevel modeling with the R package brms. R J. 10, 395–411 (2018).
Acknowledgements
We thank J. H. Brown for suggestions on the manuscript. We thank T. J. McKinley for providing feedback on the statistical analysis, and P. Goncalves for assistance with illustrations. This work was supported by an AXA Research Fund (to M.D.), by the Natural Environment Research Council (NE/H022511/1, to M.T., G.Y.-D. and G.W.), and by a European Research Council grant (ERC StG 677278 TEMPDEP, to G.Y.-D.).
Author information
Authors and Affiliations
Contributions
G.Y.-D. and M.T. conceived the study; C.J.H. and M.D. collected the data and performed the stable isotope analysis; D.P. collected the phytoplankton community data from 2016; D.R.B., G.Y.-D. and M.T. conducted the statistical analyses; D.R.B. and G.Y.-D. wrote the first version of the manuscript, and all authors contributed substantially to the revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Robert Hall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Schematic of experimental pond set-up and 15N tracer measurements.
a, Twenty artificial ponds, with 10 warmed (red) by 4 °C above (since September 2006) the temperature of 10 ambient (blue) ponds, were paired in a randomized block design. b, Ponds were controlled using two temperature sensors, a heating element (HE) a thermostat (T-stat) and a solid-state relay (SSR). c, Timeline of experimental measurements, including quantification of baseline 15N% of phytoplankton and zooplankton before the addition of the K15NO3 tracer, followed by continuous sampling of excess 15N% relative to the baseline on each pond. d, Dissolved oxygen (DO) saturation (left) and pH (right) did not change before and after the addition of the tracer (see ref. 13 for measurement details). Ambient, blue triangles; warmed, red inverted triangles.
Extended Data Fig. 2 Concentration of dissolved inorganic nitrogen species in the ponds before and after the addition of the 15N tracer on 16 July 2013.
Addition of the 15N tracer had no discernible effect on the natural concentration of dissolved inorganic nitrogen in the ponds (Supplementary Table 1). Points are treatment-level means, error bars are 95% confidence intervals. The dashed lines mark 16 July 2013.
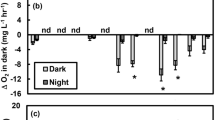
Extended Data Fig. 3 Daytime CO2 influx before and after the addition of the 15N tracer on 16 July 2013.
Each point represents an individual measurement within a pond (n = 56 measurements per treatment per period; as described in detail in ref. 13). Ambient, blue triangles; warmed, red inverted triangles. ‘Before’ measurements were taken daily throughout the week leading to the addition of the 15N tracer on 16 July 2013 (9 July–15 July), whereas ‘After’ measurements were taken daily throughout the week after the addition of the tracer (17 July–23 July). Box plots depict the median (centre line) and the first and third quartiles (lower and upper bounds). Whiskers extend to 1.5 times the the interquartile range (the distance between the first and third quartiles). Outliers were removed from the plot for visualization purposes only. A before–after analysis (see Supplementary Table 1) revealed no substantial changes in daytime CO2 influx and net primary production due to the addition of the 15N tracer.
Extended Data Fig. 4 Hierarchical structure outlining the model fitting.
Data, processes and parameters are explicitly identified, with equation (1) parameters ϕ, κa and κe being fitted at the treatment level with pond-level deviations. Phytoplankton and zooplankton silhouettes depict whether a certain transformation or prior was used for either group or both (see Methods).
Extended Data Fig. 5 Temporal dynamics of excess 15N%, χ, in phytoplankton and zooplankton during the experiment.
Dashed lines represent mean pond-level predictions that were obtained by fitting the data to equation (1) via a nonlinear hierarchical Bayesian model (see Methods). Shaded polygons represent Bayesian 95% CI that were calculated from 20,000 posterior draws. Note the sharp increase in the χ(t) in the first few days of the experiment, particularly when compared to baseline 15N% in the control ponds (Extended Data Fig. 7).
Extended Data Fig. 6 Posterior distributions of percentage decline in carbon biomass (μg C l−1) and efficiency of nitrogen transfer due to long-term warming.
Distributions were calculated using 20,000 posterior draws that were estimated via Bayesian hierarchical linear models (see Methods). Positive and negative values represent percentage decline and increase, respectively. The strong overlap between distributions corroborates the assumption that mean nitrogen transfer efficiency, \(\bar{\varepsilon }\), as calculated from the 15N tracer dynamics (equation (3)), reflects the efficiency of carbon transfer and hence energy transfer.
Extended Data Fig. 7 Measurements of excess 15N%, χ, in three untreated control ponds.
Green circles represent phytoplankton (n = 5 per pond), whereas brown squares represent zooplankton (n = 3–5 per pond). These results are expected given that no 15N tracer was added. The y axis was kept fixed in order to compare the magnitude of change between treatments (Extended Data Fig. 5) and controls. For further explanations of how the data were collected, see Methods.
Extended Data Fig. 8 Effects of long-term warming on mean nitrogen biomass.
Mean biomass nitrogen estimates were calculated from ambient and warmed ponds. Points represent means calculated for the entire duration of the 15N tracer experiment (n = 8 per treatment). Box plots depict the median (centre line) and the first and third quartiles (lower and upper bounds). Whiskers extend to 1.5 times the the interquartile range (the distance between the first and third quartiles). Circles represent phytoplankton (top) and squares represent zooplankton (bottom).
Extended Data Fig. 9 Effects of long-term warming on C:N ratios.
Mean C:N ratios were calculated in ambient and warmed ponds. Points represent means calculated for the entire duration of the experiment (n = 8 per treatment). Box plots depict the median (centre line) and the first and third quartiles (lower and upper bounds). Whiskers extend to 1.5 times the the interquartile range (the distance between the first and third quartiles). Circles represent phytoplankton (top) and squares represent zooplankton (bottom).
Supplementary information
Supplementary Information
This file contains the Empirical Proof of Concept, Supplementary Figures 1- 13, Supplementary Tables 1-2 and Supplementary References.
Rights and permissions
About this article
Cite this article
Barneche, D.R., Hulatt, C.J., Dossena, M. et al. Warming impairs trophic transfer efficiency in a long-term field experiment. Nature 592, 76–79 (2021). https://doi.org/10.1038/s41586-021-03352-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03352-2
This article is cited by
-
Steeper size spectra with decreasing phytoplankton biomass indicate strong trophic amplification and future fish declines
Nature Communications (2024)
-
Disruption of ecological networks in lakes by climate change and nutrient fluctuations
Nature Climate Change (2023)
-
What the geological past can tell us about the future of the ocean’s twilight zone
Nature Communications (2023)
-
Direct biological fixation provides a freshwater sink for N2O
Nature Communications (2023)
-
Stocks of soil carbon, nitrogen, and phosphorus in coniferous forests on the Qilian Mountains: spatial trends and drivers
European Journal of Forest Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.