Abstract
Sleep remains a major mystery of biology, with little understood about its basic function. One of the most commonly proposed functions of sleep is the consolidation of memory1,2,3. However, as conditions such as starvation require the organism to be awake and active4, the ability to switch to a memory consolidation mechanism that is not contingent on sleep may confer an evolutionary advantage. Here we identify an adaptive circuit-based mechanism that enables Drosophila to form sleep-dependent and sleep-independent memory. Flies fed after appetitive conditioning needed increased sleep for memory consolidation, but flies starved after training did not require sleep to form memories. Memory in fed flies is mediated by the anterior–posterior α′/β′ neurons of the mushroom body, while memory under starvation is mediated by medial α′/β′ neurons. Sleep-dependent and sleep-independent memory rely on distinct dopaminergic neurons and corresponding mushroom body output neurons. However, sleep and memory are coupled such that mushroom body neurons required for sleep-dependent memory also promote sleep. Flies lacking Neuropeptide F display sleep-dependent memory even when starved, suggesting that circuit selection is determined by hunger. This plasticity in memory circuits enables flies to retain essential information in changing environments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Source data are provided with this paper.
References
Le Glou, E., Seugnet, L., Shaw, P. J., Preat, T. & Goguel, V. Circadian modulation of consolidated memory retrieval following sleep deprivation in Drosophila. Sleep 35, 1377–1384 (2012).
Rasch, B. & Born, J. About sleep’s role in memory. Physiol. Rev. 93, 681–766 (2013).
Ganguly-Fitzgerald, I., Donlea, J. & Shaw, P. J. Waking experience affects sleep need in Drosophila. Science 313, 1775–1781 (2006).
Keene, A. C. et al. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr. Biol. 20, 1209–1215 (2010).
Krashes, M. J. & Waddell, S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J. Neurosci. 28, 3103–3113 (2008).
Shi, M., Yue, Z., Kuryatov, A., Lindstrom, J. M. & Sehgal, A. Identification of Redeye, a new sleep-regulating protein whose expression is modulated by sleep amount. eLife 3, e01473 (2014).
Wigglesworth, V. B. The utilization of reserve substances in Drosophila during flight. J. Exp. Biol. 26, 150–163 (1949).
Burke, C. J. & Waddell, S. Remembering nutrient quality of sugar in Drosophila. Curr. Biol. 21, 746–750 (2011).
Wu, Q. et al. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron 39, 147–161 (2003).
Wu, Q., Zhao, Z. & Shen, P. Regulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systems. Nat. Neurosci. 8, 1350–1355 (2005).
Krashes, M. J. et al. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, 416–427 (2009).
Lin, H.-H., Lai, J. S.-Y., Chin, A.-L., Chen, Y.-C. & Chiang, A.-S. A map of olfactory representation in the Drosophila mushroom body. Cell 128, 1205–1217 (2007).
Strausfeld, N. J., Sinakevitch, I. & Vilinsky, I. The mushroom bodies of Drosophila melanogaster: an immunocytological and golgi study of Kenyon cell organization in the calyces and lobes. Microsc. Res. Tech. 62, 151–169 (2003).
Aso, Y. et al. The neuronal architecture of the mushroom body provides a logic for associative learning. eLife 3, e04577 (2014).
Yang, C.-H. et al. Additive expression of consolidated memory through Drosophila mushroom body subsets. PLoS Genet. 12, e1006061 (2016).
Haynes, P. Functional and Anatomical Interactions of Sleep- and Memory Consolidation-Promoting Circuitry in Drosophila. PhD thesis, Brandeis Univ. (2015).
Masuyama, K., Zhang, Y., Rao, Y. & Wang, J. W. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J. Neurogenet. 26, 89–102 (2012).
Haynes, P. R., Christmann, B. L. & Griffith, L. C. A single pair of neurons links sleep to memory consolidation in Drosophila melanogaster. eLife 4, e03868 (2015).
Sitaraman, D. et al. Propagation of homeostatic sleep signals by segregated synaptic microcircuits of the Drosophila mushroom body. Curr. Biol. 25, 2915–2927 (2015).
Musso, P.-Y., Tchenio, P. & Preat, T. Delayed dopamine signaling of energy level builds appetitive long-term memory in Drosophila. Cell Rep. 10, 1023–1031 (2015).
Aso, Y. & Rubin, G. M. Dopaminergic neurons write and update memories with cell-type-specific rules. eLife 5, e16135 (2016).
Aso, Y. et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. eLife 3, e04580 (2014).
Berry, J. A., Phan, A. & Davis, R. L. Dopamine neurons mediate learning and forgetting through bidirectional modulation of a memory trace. Cell Rep. 25, 651–662 (2018).
Felsenberg, J., Barnstedt, O., Cognigni, P., Lin, S. & Waddell, S. Re-evaluation of learned information in Drosophila. Nature 544, 240–244 (2017).
Pavlowsky, A., Schor, J., Plaçais, P.-Y. & Preat, T. A GABAergic feedback shapes dopaminergic input on the Drosophila mushroom body to promote appetitive long-term memory. Curr. Biol. 28, 1783–1793 (2018).
Graves, L. A., Heller, E. A., Pack, A. I. & Abel, T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn. Mem. 10, 168–176 (2003).
Weber, F. D., Wang, J.-Y., Born, J. & Inostroza, M. Sleep benefits in parallel implicit and explicit measures of episodic memory. Learn. Mem. 21, 190–198 (2014).
van der Helm, E., Gujar, N., Nishida, M. & Walker, M. P. Sleep-dependent facilitation of episodic memory details. PLoS ONE 6, e27421 (2011).
Aly, M. & Moscovitch, M. The effects of sleep on episodic memory in older and younger adults. Memory 18, 327–334 (2010).
Colomb, J., Kaiser, L., Chabaud, M.-A. & Preat, T. Parametric and genetic analysis of Drosophila appetitive long-term memory and sugar motivation. Genes Brain Behav. 8, 407–415 (2009).
Hendricks, J. C. et al. Rest in Drosophila is a sleep-like state. Neuron 25, 129–138 (2000).
Shaw, P. J., Cirelli, C., Greenspan, R. J. & Tononi, G. Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 (2000).
Lenz, O., Xiong, J., Nelson, M. D., Raizen, D. M. & Williams, J. A. FMRFamide signaling promotes stress-induced sleep in Drosophila. Brain Behav. Immun. 47, 141–148 (2015).
Acknowledgements
We thank the members of the Sehgal laboratory for helpful discussions, and K. Lin for input on statistics. We thank C. Stein and A. Kolesnik for their assistance in imaging experiments. This work was supported by R01 DK120757 (to A.S.) and by the HHMI. P.H. was supported by T32 MH014654. Initial studies were supported by R01 MH067284 (to L.C.G.).
Author information
Authors and Affiliations
Contributions
N.S.C., P.H., L.C.G. and A.S. conceived the project. N.S.C. and A.S. designed all experiments. P.H. conducted pilot sleep-deprivation experiments and identified the sleep-promoting role of α′/β′ap neurons, N.S.C. conducted and analysed all behavioural experiments. N.S.C. and P.H. conducted and analysed imaging experiments. The manuscript was written by N.S.C. and A.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Ravi Allada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
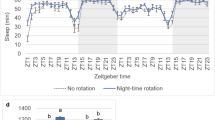
Extended Data Fig. 1 Sleep increases in flies fed after appetitive training.
(a) Flies starved for 6 h before training show no difference in sleep between trained and untrained groups. However, moving trained flies into sucrose tubes post-training resulted in a significant increase in sleep compared to untrained controls despite only 6 h of pre-training starvation. Sleep was quantified for the ZT8-12 interval (two-sided t-tests were performed for each condition to compare trained and untrained groups, followed by Bonferroni correction; n = 32). (b) and (c) Training increases sleep bout length in fed flies but not in starved flies. Flies were trained after 18 h (b) and 6 h (c) starvation (two-sided Mann–Whitney U-tests were performed for each condition to compare trained and untrained groups; n = 32). Data are represented as mean ± s.e.m. Each data point depicts a single fly. Precise ‘n’ and ‘p’ values are in the Source Data. ***P < 0.001; **P < 0.01; *P < 0.05.
Extended Data Fig. 2 Memory in flies fed after training is sleep and protein synthesis-dependent but independent of light cycles.
(a) Long-term memory in fed flies is sensitive to cycloheximide based inhibition of protein-synthesis (two-sided t-test; n ≥ 6). (b) Flies demonstrate substantial rebound sleep when sleep-deprived in a group of about 100 flies in a vial in both fed and starved conditions. Flies were sleep-deprived from ZT12-ZT24 and then introduced individually into locomotor tubes (two-sided Mann–Whitney U-tests were performed for each condition to compare undisturbed and sleep-deprived groups; n = 32). (c) Starved flies were effectively sleep-deprived when exposed to a mechanical stimulus post-training (n ≥ 31). Flies were starved for 6 h and then trained at ZT6 and subsequently introduced into agar locomotor tubes. A mechanical stimulus was applied for 6 h after training. A rebound is evident after sleep deprivation. (d) Flies starved for only 6 h, as opposed to 18 h, before training and then allowed to feed showed impaired memory performance when sleep-deprived for 6 h post-training (two-sided t-test; n = 8). Sleep post-training was comparable to flies depicted in Extended Data Fig. 1a. (e) 6 h sleep deprivation had no effect on long-term memory in flies kept starved after training. Here, flies were starved for 6 h before training (two-sided t-test; n ≥ 6). Sleep post-training was comparable to flies depicted in Extended Data Fig. 1a. (f) Sleep deprivation initiated 6 h after training had no effect on memory in fed and trained flies (two-sided t-test; n = 8). (g) Long-term memory was resistant to sleep deprivation in flies that were starved after conditioning but then tested after a feeding and re-starvation period (two-sided t-test; n ≥ 6). Flies were starved (and sleep-deprived) for 6 h post-training and then allowed to feed for 18 h before 30 h restarvation for memory tests. (h) and (i) 6 h sleep deprivation of flies maintained in constant light affected appetitive long-term memory when they were fed, but not starved, post-training (two-sided t-test; n ≥ 6). Data are represented as mean ± s.e.m. Each data point in a memory experiment represents a group of flies and in a sleep experiment it depicts a single fly. Precise ‘n’ and ‘p’ values are in the Source Data. ***P < 0.001; **P < 0.01; *P < 0.05.
Extended Data Fig. 3 The rye mutation affects sleep-dependent memory.
(a) Long-term memory is substantially lower in satiated short-sleeping rye mutants. Background iso31 line was used as control (two-sided t-test; n ≥ 8). (b) rye mutants form robust appetitive 24 h memory, similar to controls when kept starved (two-sided t-test; n ≥ 6). (c) Satiated rye mutants demonstrate no difference in sleep between trained and untrained groups. Total sleep in the ZT8-12 interval is depicted (two-sided t-tests were performed for each genotype to compare trained and untrained groups, followed by Bonferroni correction; n ≥ 31). Data are represented as mean ± s.e.m. Each data point in a memory experiment represents a group of flies and in a sleep experiment it depicts a single fly. Precise ‘n’ and ‘p’ values are in the Source Data. **P < 0.01; *P < 0.05.
Extended Data Fig. 4 Flies on arabinose demonstrate a significant increase in post-training sleep.
(a) Trained flies show a substantial increase in sleep relative to untrained flies when kept on arabinose after appetitive conditioning. Sleep was quantified for the 0–4 h interval post-training (two-sided t-test; n ≥ 31). (b) Bout length was considerably higher in trained flies compared to untrained flies when moved to arabinose after training (two-sided Mann–Whitney U-test; n ≥ 31). Data are represented as mean ± s.e.m. Each data point depicts a single fly. Precise ‘n’ and ‘p’ values are in the Source Data. ***P < 0.001; **P < 0.01.
Extended Data Fig. 5 npf signalling is essential for sleep-independent memory in starved flies.
(a) Starved npfr mutant flies show a substantial increase in sleep post-training compared to untrained flies. npfr/+ was used as control. Total sleep in the 0–4 h interval post-training is depicted (two-sided t-tests were performed for each genotype to compare trained and untrained groups, followed by Bonferroni correction; n ≥ 32). (b) Bout length was considerably higher in trained and starved npfr mutant flies compared to untrained flies. npfr/+ was used as control (two-sided Mann–Whitney U-tests were performed for each genotype to compare trained and untrained groups; n ≥ 32). (c) RNAi knockdown of npfr pan-neuronally results in sleep-dependent memory formation in hungry flies. 6 h sleep disruption post-training resulted in impaired memory performance in UAS-npfr-RNAi/n-syb-Gal4 flies (two-sided t-tests were performed for each genotype to compare undisturbed and sleep-deprived groups, followed by Bonferroni correction; n ≥ 6). (d) Starved UAS-npf-RNAi/NPF-Gal4 flies show lower long-term memory when sleep-deprived for 6 h post-training (two-sided t-tests were performed for each genotype to compare undisturbed and sleep-deprived groups, followed by Bonferroni correction; n ≥ 6). Data are represented as mean ± s.e.m. Each data point in a memory experiment represents a group of flies and in a sleep experiment it depicts a single fly. Precise ‘n’ and ‘p’ values are in the Source Data. ***P < 0.001; *P < 0.05.
Extended Data Fig. 6 α’/β’ neurotransmission is essential for long-term memory under both fed and starved conditions.
(a) Starved UAS-shibirets1/MB461B flies kept at restrictive settings for 4 h immediately after training show impaired long-term memory (n ≥ 6). Restrictive temperature 8-12 h after training had no effect (b) (n = 6) (one-factor ANOVA with Tukey post hoc test). (c) Long-term memory remained unchanged in experimental and control flies when kept at 25°C (one-factor ANOVA with Tukey post hoc test; n = 6). (d) and (e) Silencing α’/β’ neurons immediately after conditioning, but not at hours 8-12, affects long-term memory in fed flies (one-factor ANOVA with Tukey post hoc test; n ≥ 6). (f) Long-term memory remained intact in UAS-shibirets1/MB461B flies fed after training but maintained at the permissive temperature (one-factor ANOVA with Tukey post hoc test; n ≥ 6). Data are represented as mean ± s.e.m. Each data point represents a group of flies. Precise ‘n’ and ‘p’ values are in the Source Data. **P < 0.01. Asterisks in (a, d) indicate a significant difference between experimental flies and genetic controls.
Extended Data Fig. 7 Effects of manipulating the activity of α’/β’ subset specific neurons on long-term memory.
(a) and (b) Neurotransmission from α’/β’m neurons (UAS-shibirets1/R26E01 and UAS-shibirets1/MB370B) is dispensable for long-term memory in fed flies (n ≥ 6). Temperature controls are depicted in (b) (n ≥ 6) (one-factor ANOVA with Tukey post hoc test). (c) and (d) Blocking the activity of α’/β’ap neurons (UAS-shibirets1/R35B12 and UAS-shibirets1/VT50658) for 4 h after conditioning in starved flies has no effect on long-term memory (n = 6). Long-term memory in experimental and control flies at the permissive temperature, 25°C, is shown in (d) (n = 6) (one-factor ANOVA with Tukey post hoc test). (e) shibirets does not affect memory in flies maintained under starvation conditions at the permissive temperature (one-factor ANOVA with Tukey post hoc test; n = 6). Controls related to Fig. 2(a). (f) shibirets has no effect on memory in flies maintained on food at the permissive temperature (one-factor ANOVA with Tukey post hoc test; n ≥ 6). Controls related to Fig. 2(b). (g) Hyperactivation of α’/β’ap neurons (UAS-TrpA1/R35B12) for 4 h post-training does not affect long-term memory formation in starved flies (one-factor ANOVA with Tukey post hoc test; n = 6). (h) Memory was not affected in UAS-TrpA1/R35B12 flies at permissive settings (one-factor ANOVA with Tukey post hoc test; n = 6). Data are represented as mean ± s.e.m. Each data point in a memory experiment represents a group of flies. Precise ‘n’ and ‘p’ values are in the Source Data.
Extended Data Fig. 8 α’/β’ subsets differentially regulate sleep.
(a) Thermogenetic activation of α’/β’ap neurons (UAS-TrpA1/VT50658) results in a considerable enhancement in sleep while flies in which α’/β’m neurons (UAS-TrpA1/MB370B) were activated showed a significant decrease in sleep (one-factor ANOVA with Tukey post hoc test; n ≥ 30). (b) and (c) Disabling neurotransmission in α’/β’ap neurons (UAS-shibirets1/R35B12 and UAS-shibirets1/VT50658) or α’/β’m neurons (UAS-shibirets1/R26E01 and UAS-shibirets1/MB370B) had no effect on sleep (one-factor ANOVA with Tukey post hoc test; n ≥ 31). Data are represented as mean ± s.e.m. Each data point depicts a single fly. Precise ‘n’ and ‘p’ values are in the Source Data. Asterisks in (a) indicate a significant difference between experimental flies and genetic controls.
Extended Data Fig. 9 The activity of α’/β’ap, but not α’/β’m, neurons is relevant for sleep after conditioning.
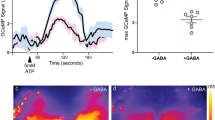
(a) shibirets expression in α’/β’ap neurons has no effect on sleep post-training if flies are maintained at the permissive temperature (two-sided t-tests were performed for each genotype to compare trained and untrained groups, followed by Bonferroni correction; n ≥ 30). Controls related to Fig. 2(g). (b) and (c) A training-dependent increase in sleep bout length was prevented in flies in which α’/β’ap neurons were silenced (n ≥ 31). Temperature controls are shown in (c) (n ≥ 30) (two-sided Mann–Whitney U-tests were performed for each genotype to compare trained and untrained groups). (d) and (e) Trained flies expressing shibirets1 in α’/β’m neurons showed an enhancement in sleep even when moved to 32°C for 4 h post-training. The total amount of sleep in 0–4 h interval after training is quantified (n ≥ 32). Post-training sleep in experimental and control flies at the permissive temperature, 25°C, is shown in (e) (n ≥ 16) (two-sided t-tests were performed for each genotype to compare trained and untrained groups, followed by Bonferroni correction). (f) and (g) Silencing α’/β’m neurons does not prevent an increase in sleep bout length after training (n ≥ 32). Temperature controls are shown in (g) (n ≥ 16) (two-sided Mann–Whitney U-tests were performed for each genotype to compare trained and untrained groups). (h) Calcium/GFP signal in α’/β’m neurons was comparable between control and sleep-deprived flies when kept starved post-training (two-sided Mann–Whitney U-test; n ≥ 11). Representative images are shown, two independent experiments; Scale bar, 50 μm. Data are represented as mean ± s.e.m. Each data point depicts a single fly. Precise ‘n’ and ‘p’ values are in the Source Data. ***P < 0.001; **P < 0.01; *P < 0.05.
Extended Data Fig. 10 Effects of manipulating the neurotransmission of PPL1 neurons and MBONs on long-term memory.
(a) and (b) Trained starved flies show lower long-term memory performance when the PPL1 cluster neurons (UAS-shibirets1/MB504B) are silenced for 4 h post-training (n ≥ 8). Temperature controls are shown in (b) (n ≥ 7) (one-factor ANOVA with Tukey post hoc test). (c) and (d) Silencing PPL1 DANs affects long-term memory performance in flies kept fed after training (n ≥ 7). Temperature controls are shown in (d) (n ≥ 6) (one-factor ANOVA with Tukey post hoc test). (e) Expression of shibirets1 in MP1 and MV1 neurons at permissive temperature does not affect memory in flies starved after training (one-factor ANOVA with Tukey post hoc test; n ≥ 6). Controls related to Fig. 3a. (f) Permissive temperature control for Fig. 3b. Expression of shibirets1 in MP1 and MV1 neurons does not affect memory in flies kept on food at 25°C after training (one-factor ANOVA with Tukey post hoc test; n ≥ 6). (g) Blocking the activity of MP1 neurons (UAS-shibirets1/MB320C at restrictive temperature) for 6 h after conditioning has no effect on long-term memory in flies kept on food vials after training (one-factor ANOVA with Tukey post hoc test; n = 6). (h) and (i) Long-term memory in UAS-shibirets1/MB077B and UAS-shibirets1/MB112C flies was similar to that of genetic controls when kept starved or fed at 25°C (one-factor ANOVA with Tukey post hoc test; n ≥ 6). Temperature controls related to Fig. 3c, d. Data are represented as mean ± s.e.m. Each data point represents a group of flies. Precise ‘n’ and ‘p’ values are in the Source Data. Asterisks in (a, c) indicate a significant difference between experimental flies and genetic controls.
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
Chouhan, N.S., Griffith, L.C., Haynes, P. et al. Availability of food determines the need for sleep in memory consolidation. Nature 589, 582–585 (2021). https://doi.org/10.1038/s41586-020-2997-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2997-y
This article is cited by
-
Acetylcholine deficit causes dysfunctional inhibitory control in an aging-dependent manner
Scientific Reports (2022)
-
A brain signal that coordinates thought with metabolism
Nature (2021)
-
Flies sense the world while sleeping
Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.