Abstract
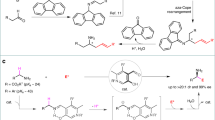
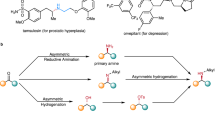
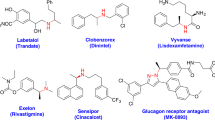
Hydroamination of alkenes, the addition of the N–H bond of an amine across an alkene, is a fundamental, yet challenging, organic transformation that creates an alkylamine from two abundant chemical feedstocks, alkenes and amines, with full atom economy1,2,3. The reaction is particularly important because amines, especially chiral amines, are prevalent substructures in a wide range of natural products and drugs. Although extensive efforts have been dedicated to developing catalysts for hydroamination, the vast majority of alkenes that undergo intermolecular hydroamination have been limited to conjugated, strained, or terminal alkenes2,3,4; only a few examples occur by the direct addition of the N–H bond of amines across unactivated internal alkenes5,6,7, including photocatalytic hydroamination8,9, and no asymmetric intermolecular additions to such alkenes are known. In fact, current examples of direct, enantioselective intermolecular hydroamination of any type of unactivated alkene lacking a directing group occur with only moderate enantioselectivity10,11,12,13. Here we report a cationic iridium system that catalyses intermolecular hydroamination of a range of unactivated, internal alkenes, including those in both acyclic and cyclic alkenes, to afford chiral amines with high enantioselectivity. The catalyst contains a phosphine ligand bearing trimethylsilyl-substituted aryl groups and a triflimide counteranion, and the reaction design includes 2-amino-6-methylpyridine as the amine to enhance the rates of multiple steps within the catalytic cycle while serving as an ammonia surrogate. These design principles point the way to the addition of N–H bonds of other reagents, as well as O–H and C–H bonds, across unactivated internal alkenes to streamline the synthesis of functional molecules from basic feedstocks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information.
References
Müller, T. E. & Beller, M. Metal-initiated amination of alkenes and alkynes. Chem. Rev. 98, 675–704 (1998).
Müller, T. E., Hultzsch, K. C., Yus, M., Foubelo, F. & Tada, M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem. Rev. 108, 3795–3892 (2008).
Huang, L., Arndt, M., Gooßen, K., Heydt, H. & Gooßen, L. J. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 115, 2596–2697 (2015).
Reznichenko, A. L. & Hultzsch, K. C. in Organic Reactions 1–554 (Wiley, 2015).
Gurak, J. A., Yang, K. S., Liu, Z. & Engle, K. M. Directed, regiocontrolled hydroamination of unactivated alkenes via protodepalladation. J. Am. Chem. Soc. 138, 5805–5808 (2016).
Karshtedt, D., Bell, A. T. & Tilley, T. D. Platinum-based catalysts for the hydroamination of olefins with sulfonamides and weakly basic anilines. J. Am. Chem. Soc. 127, 12640–12646 (2005).
Zhang, J., Yang, C.-G. & He, C. Gold(i)-catalyzed intra- and intermolecular hydroamination of unactivated olefins. J. Am. Chem. Soc. 128, 1798–1799 (2006).
Musacchio, A. J. et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science 355, 727–730 (2017).
Nguyen, T. M., Manohar, N. & Nicewicz, D. A. anti-Markovnikov hydroamination of alkenes catalyzed by a two-component organic photoredox system: direct access to phenethylamine derivatives. Angew. Chem. Int. Ed. 53, 6198–6201 (2014).
Zhang, Z., Lee, S. D. & Widenhoefer, R. A. Intermolecular hydroamination of ethylene and 1-alkenes with cyclic ureas catalyzed by achiral and chiral gold(i) complexes. J. Am. Chem. Soc. 131, 5372–5373 (2009).
Reznichenko, A. L., Nguyen, H. N. & Hultzsch, K. C. Asymmetric intermolecular hydroamination of unactivated alkenes with simple amines. Angew. Chem. Int. Ed. 49, 8984–8987 (2010).
Pan, S., Endo, K. & Shibata, T. Ir(i)-catalyzed intermolecular regio- and enantioselective hydroamination of alkenes with heteroaromatic amines. Org. Lett. 14, 780–783 (2012).
Vanable, E. P. et al. Rhodium-catalyzed asymmetric hydroamination of allyl amines. J. Am. Chem. Soc. 141, 739–742 (2019).
Nugent, T. C. Chiral Amine Synthesis: Methods, Developments and Applications (Wiley VCH, 2010).
Trowbridge, A., Walton, S. M. & Gaunt, M. J. New strategies for the transition-metal catalyzed synthesis of aliphatic amines. Chem. Rev. 120, 2613–2692 (2020).
Wang, C. & Xiao, J. in Stereoselective Formation of Amines (eds Li, W. & Zhang, X.) 261–282 (Springer, 2014).
Nugent, T. C. & El-Shazly, M. Chiral amine synthesis – recent developments and trends for enamide reduction, reductive amination, and imine reduction. Adv. Synth. Catal. 352, 753–819 (2010).
Patil, M. D., Grogan, G., Bommarius, A. & Yun, H. Oxidoreductase-catalyzed synthesis of chiral amines. ACS Catal. 8, 10985–11015 (2018).
Xie, J.-H., Zhu, S.-F. & Zhou, Q.-L. Transition metal-catalyzed enantioselective hydrogenation of enamines and imines. Chem. Rev. 111, 1713–1760 (2011).
Ellman, J. A., Owens, T. D. & Tang, T. P. N-tert-Butanesulfinyl imines: versatile intermediates for the asymmetric synthesis of amines. Acc. Chem. Res. 35, 984–995 (2002).
You, S.-L., Zhu, X.-Z., Luo, Y.-M., Hou, X.-L. & Dai, L.-X. Highly regio- and enantioselective Pd-catalyzed allylic alkylation and amination of monosubstituted allylic acetates with novel ferrocene P,N-ligands. J. Am. Chem. Soc. 123, 7471–7472 (2001).
Ohmura, T. & Hartwig, J. F. Regio- and enantioselective allylic amination of achiral allylic esters catalyzed by an iridium−phosphoramidite complex. J. Am. Chem. Soc. 124, 15164–15165 (2002).
Löber, O., Kawatsura, M. & Hartwig, J. F. Palladium-catalyzed hydroamination of 1,3-dienes: a colorimetric assay and enantioselective additions. J. Am. Chem. Soc. 123, 4366–4367 (2001).
Adamson, N. J., Hull, E. & Malcolmson, S. J. Enantioselective intermolecular addition of aliphatic amines to acyclic dienes with a Pd–PHOX catalyst. J. Am. Chem. Soc. 139, 7180–7183 (2017).
Long, J., Wang, P., Wang, W., Li, Y. & Yin, G. Nickel/Brønsted acid-catalyzed chemo- and enantioselective intermolecular hydroamination of conjugated dienes. iScience 22, 369–379 (2019).
Tran, G., Shao, W. & Mazet, C. Ni-catalyzed enantioselective intermolecular hydroamination of branched 1,3-dienes using primary aliphatic amines. J. Am. Chem. Soc. 141, 14814–14822 (2019).
Kawatsura, M. & Hartwig, J. F. Palladium-catalyzed intermolecular hydroamination of vinylarenes using arylamines. J. Am. Chem. Soc. 122, 9546–9547 (2000).
Utsunomiya, M. & Hartwig, J. F. Intermolecular, Markovnikov hydroamination of vinylarenes with alkylamines. J. Am. Chem. Soc. 125, 14286–14287 (2003).
Yang, Y., Shi, S.-L., Niu, D., Liu, P. & Buchwald, S. L. Catalytic asymmetric hydroamination of unactivated internal olefins to aliphatic amines. Science 349, 62–66 (2015).
Gui, J. et al. Practical olefin hydroamination with nitroarenes. Science 348, 886–891 (2015).
Johns, A. M., Sakai, N., Ridder, A. & Hartwig, J. F. Direct measurement of the thermodynamics of vinylarene hydroamination. J. Am. Chem. Soc. 128, 9306–9307 (2006).
Liu, Z. & Hartwig, J. F. Mild, rhodium-catalyzed intramolecular hydroamination of unactivated terminal and internal alkenes with primary and secondary amines. J. Am. Chem. Soc. 130, 1570–1571 (2008).
Huang, J.-M., Wong, C.-M., Xu, F.-X. & Loh, T.-P. InBr3 catalyzed intermolecular hydroamination of unactivated alkenes. Tetrahedron Lett. 48, 3375–3377 (2007).
Sevov, C. S., Zhou, J. & Hartwig, J. F. Iridium-catalyzed intermolecular hydroamination of unactivated aliphatic alkenes with amides and sulfonamides. J. Am. Chem. Soc. 134, 11960–11963 (2012).
Utsunomiya, M., Kuwano, R., Kawatsura, M. & Hartwig, J. F. Rhodium-catalyzed anti-Markovnikov hydroamination of vinylarenes. J. Am. Chem. Soc. 125, 5608–5609 (2003).
Pawlas, J., Nakao, Y., Kawatsura, M. & Hartwig, J. F. A general nickel-catalyzed hydroamination of 1,3-dienes by alkylamines: catalyst selection, scope, and mechanism. J. Am. Chem. Soc. 124, 3669–3679 (2002).
Casalnuovo, A. L., Calabrese, J. C. & Milstein, D. Rational design in homogeneous catalysis. Iridium(i)-catalyzed addition of aniline to norbornylene via nitrogen-hydrogen activation. J. Am. Chem. Soc. 110, 6738–6744 (1988).
Dorta, R., Egli, P., Zürcher, F. & Togni, A. The [IrCl(diphosphine)]2/fluoride system. Developing catalytic asymmetric olefin hydroamination. J. Am. Chem. Soc. 119, 10857–10858 (1997).
Zhou, J. & Hartwig, J. F. Intermolecular, catalytic asymmetric hydroamination of bicyclic alkenes and dienes in high yield and enantioselectivity. J. Am. Chem. Soc. 130, 12220–12221 (2008).
Sevov, C. S., Zhou, J. & Hartwig, J. F. Iridium-catalyzed, intermolecular hydroamination of unactivated alkenes with indoles. J. Am. Chem. Soc. 136, 3200–3207 (2014).
Hanley, P. S. & Hartwig, J. F. Migratory insertion of alkenes into metal–oxygen and metal–nitrogen bonds. Angew. Chem. Int. Ed. 52, 8510–8525 (2013).
Thompson, W. H. & Sears, C. T. Kinetics of oxidative addition to iridium(i) complexes. Inorg. Chem. 16, 769–774 (1977).
Smout, V. et al. Removal of the pyridine directing group from α-substituted N-(pyridin-2-yl)piperidines obtained via directed Ru-catalyzed sp3 C–H functionalization. J. Org. Chem. 78, 9803–9814 (2013).
Hanley, P. S. & Hartwig, J. F. Intermolecular migratory insertion of unactivated olefins into palladium–nitrogen bonds. Steric and electronic effects on the rate of migratory insertion. J. Am. Chem. Soc. 133, 15661–15673 (2011).
Zhang, M., Hu, L., Lang, Y., Cao, Y. & Huang, G. Mechanism and origins of regio- and enantioselectivities of iridium-catalyzed hydroarylation of alkenyl ethers. J. Org. Chem. 83, 2937–2947 (2018).
Xing, D., Qi, X., Marchant, D., Liu, P. & Dong, G. Branched-selective direct α-alkylation of cyclic ketones with simple alkenes. Angew. Chem. Int. Ed. 58, 4366–4370 (2019).
Xi, Y., Butcher, T. W., Zhang, J. & Hartwig, J. F. Regioselective, asymmetric formal hydroamination of unactivated internal alkenes. Angew. Chem. Int. Ed. 55, 776–780 (2016).
Mei, T.-S., Werner, E. W., Burckle, A. J. & Sigman, M. S. Enantioselective redox-relay oxidative heck arylations of acyclic alkenyl alcohols using boronic acids. J. Am. Chem. Soc. 135, 6830–6833 (2013).
Morandi, B., Wickens, Z. K. & Grubbs, R. H. Regioselective Wacker oxidation of internal alkenes: rapid access to functionalized ketones facilitated by cross-metathesis. Angew. Chem. Int. Ed. 52, 9751–9754 (2013).
Acknowledgements
The enantioselective aspects of the work were supported by the National Institutes of Health under grant R35GM130387 and the catalyst development was supported by the Director, Office of Science, of the US Department of Energy under contract number DE-AC02-05CH11231. Calculations were performed at the Molecular Graphics and Computation Facility at UC Berkeley funded by the NIH (S10OD023532). We gratefully acknowledge Takasago for gifts of (S)-DTBM-SEGPHOS, and H. Celik for assistance with nuclear magnetic resonance (NMR) experiments. Instruments in the College of Chemistry NMR facility are supported in part by NIH S10OD024998. We thank R. G. Bergman, B. Su and T. Butcher for discussions. Y.X. thanks Bristol-Myers Squibb for a graduate fellowship, S. Pedram for supply of NaBARF and D. Small for assistance with DFT calculations.
Author information
Authors and Affiliations
Contributions
Y.X. and J.F.H. conceived the project. Y.X. discovered the reaction and performed experiments and DFT calculations. S.M. performed experiments for revision. Y.X. and J.F.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains experimental protocols and spectral data – see contents for details.
Rights and permissions
About this article
Cite this article
Xi, Y., Ma, S. & Hartwig, J.F. Catalytic asymmetric addition of an amine N–H bond across internal alkenes. Nature 588, 254–260 (2020). https://doi.org/10.1038/s41586-020-2919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2919-z
This article is cited by
-
Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles
Nature Chemistry (2024)
-
Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines
Nature (2023)
-
Regio- and enantioselective remote dioxygenation of internal alkenes
Nature Chemistry (2023)
-
Enantioselective synthesis of α-aminoboronates by NiH-catalysed asymmetric hydroamidation of alkenyl boronates
Nature Communications (2022)
-
Development of highly efficient platinum catalysts for hydroalkoxylation and hydroamination of unactivated alkenes
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.