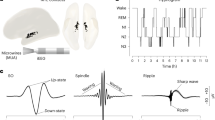
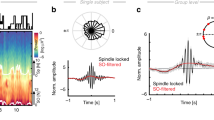
Abstract
The hippocampus has a major role in encoding and consolidating long-term memories, and undergoes plastic changes during sleep1. These changes require precise homeostatic control by subcortical neuromodulatory structures2. The underlying mechanisms of this phenomenon, however, remain unknown. Here, using multi-structure recordings in macaque monkeys, we show that the brainstem transiently modulates hippocampal network events through phasic pontine waves known as pontogeniculooccipital waves (PGO waves). Two physiologically distinct types of PGO wave appear to occur sequentially, selectively influencing high-frequency ripples and low-frequency theta events, respectively. The two types of PGO wave are associated with opposite hippocampal spike-field coupling, prompting periods of high neural synchrony of neural populations during periods of ripple and theta instances. The coupling between PGO waves and ripples, classically associated with distinct sleep stages, supports the notion that a global coordination mechanism of hippocampal sleep dynamics by cholinergic pontine transients may promote systems and synaptic memory consolidation as well as synaptic homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available from the corresponding author upon reasonable request.
Code availability
All analyses reported in this work were made with custom code written in MATLAB (The MathWorks), and will be available from the corresponding author upon reasonable request.
Change history
09 December 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41586-020-03068-9.
References
Stickgold, R. & Walker, M. P. Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci. 28, 408–415 (2005).
Pace-Schott, E. F. & Hobson, J. A. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 3, 591–605 (2002).
Buzsáki, G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015).
Grosmark, A. D., Mizuseki, K., Pastalkova, E., Diba, K. & Buzsáki, G. REM sleep reorganizes hippocampal excitability. Neuron 75, 1001–1007 (2012).
Vertes, R. P. Brain stem generation of the hippocampal EEG. Prog. Neurobiol. 19, 159–186 (1982).
Girardeau, G., Benchenane, K., Wiener, S. I., Buzsáki, G. & Zugaro, M. B. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12, 1222–1223 (2009).
Green, J. D. & Arduini, A. A. Hippocampal electrical activity in arousal. J. Neurophysiol. 17, 533–557 (1954).
Montgomery, S. M., Sirota, A. & Buzsáki, G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J. Neurosci. 28, 6731–6741 (2008).
Tononi, G. & Cirelli, C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34 (2014).
Logothetis, N. K. et al. Hippocampal–cortical interaction during periods of subcortical silence. Nature 491, 547–553 (2012).
Datta, S. Cellular basis of pontine ponto–geniculo–occipital wave generation and modulation. Cell. Mol. Neurobiol. 17, 341–365 (1997).
Jouvet, M. Recherches sur les structures nerveuses et les mecanismes responsables des differentes phases du sommeil physiologique (Research on the neural structures and responsible mechanisms in different phases of physiological sleep). Arch. Ital. Biol. 100, 125–206 (1962).
Fernández-Mendoza, J. et al. Evidence of subthalamic PGO-like waves during REM sleep in humans: a deep brain polysomnographic study. Sleep 32, 1117–1126 (2009).
Vuillon-Cacciuttolo, G. & Seri, B. Effets de la section des nerfs optiques chez le babouin sur l'activite a type de pointes genouillees et corticales au cours des divers etats de vigilance (Effects of optic nerve section in baboons on the geniculate and cortical spike activity during various states of vigilance). Electroencephalogr. Clin. Neurophysiol. 44, 754–768 (1978).
Mavanji, V. & Datta, S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: a mechanism for sleep-dependent plasticity. Eur. J. Neurosci. 17, 359–370 (2003).
Datta, S. & O’Malley, M. W. Fear extinction memory consolidation requires potentiation of pontine-wave activity during REM sleep. J. Neurosci. 33, 4561–4569 (2013).
Mavanji, V., Ulloor, J., Saha, S. & Datta, S. Neurotoxic lesions of phasic pontine-wave generator cells impair retention of 2-way active avoidance memory. Sleep 27, 1282–1292 (2004).
Hobson, J. A. REM sleep and dreaming: towards a theory of protoconsciousness. Nat. Rev. Neurosci. 10, 803–813 (2009).
Boyce, R., Glasgow, S. D., Williams, S. & Adamantidis, A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816 (2016).
Buzsáki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002).
Karashima, A., Nakao, M., Katayama, N. & Honda, K. Instantaneous acceleration and amplification of hippocampal theta wave coincident with phasic pontine activities during REM sleep. Brain Res. 1051, 50–56 (2005).
Jutras, M. J., Fries, P. & Buffalo, E. A. Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proc. Natl Acad. Sci. USA 110, 13144–13149 (2013).
Bohbot, V. D., Copara, M. S., Gotman, J. & Ekstrom, A. D. Low-frequency theta oscillations in the human hippocampus during real-world and virtual navigation. Nat. Commun. 8, 14415 (2017).
Ramirez-Villegas, J. F., Logothetis, N. K. & Besserve, M. Diversity of sharp-wave-ripple LFP signatures reveals differentiated brain-wide dynamical events. Proc. Natl Acad. Sci. USA 112, E6379–E6387 (2015).
Carr, M. F., Karlsson, M. P. & Frank, L. M. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75, 700–713 (2012).
Calvo, J. M. & Fernández-Guardiola, A. Phasic activity of the basolateral amygdala, cingulate gyrus, and hippocampus during REM sleep in the cat. Sleep 7, 202–210 (1984).
Amzica, F. & Steriade, M. Progressive cortical synchronization of ponto–geniculo–occipital potentials during rapid eye movement sleep. Neuroscience 72, 309–314 (1996).
Bowker, R. M. Variability in the characteristics of pontogeniculooccipital spikes during paradoxical sleep. Exp. Neurol. 87, 212–224 (1985).
Datta, S., Calvo, J. M., Quattrochi, J. & Hobson, J. A. Cholinergic microstimulation of the peribrachial nucleus in the cat. I. Immediate and prolonged increases in ponto–geniculo–occipital waves. Arch. Ital. Biol. 130, 263–284 (1992).
Morrison, A. R. & Pompeiano, O. Vestibular influences during sleep. IV. Functional relations between vestibular nuclei and lateral geniculate nucleus during desynchronized sleep. Arch. Ital. Biol. 104, 425–458 (1966).
Datta, S. Activation of phasic pontine-wave generator: a mechanism for sleep-dependent memory processing. Sleep Biol. Rhythms 4, 16–26 (2006).
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Shajarisales, N., Janzing, D., Schoelkopf, B. & Besserve, M. Telling cause from effect in deterministic linear dynamical systems. In Proc. 32nd International Conference on Machine Learning Vol. 37 (eds F. Bach & D. Blei) 285–294 (Proceedings of Machine Learning Research, 2015).
Hsieh, K.-C., Robinson, E. L. & Fuller, C. A. Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep 31, 1239–1250 (2008).
Fernández-Ruiz, A. et al. Long-duration hippocampal sharp wave ripples improve memory. Science 364, 1082–1086 (2019).
Griffin, A. L., Asaka, Y., Darling, R. D. & Berry, S. D. Theta-contingent trial presentation accelerates learning rate and enhances hippocampal plasticity during trace eyeblink conditioning. Behav. Neurosci. 118, 403–411 (2004).
Huerta, P. T. & Lisman, J. E. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron 15, 1053–1063 (1995).
Poe, G. R., Nitz, D. A., McNaughton, B. L. & Barnes, C. A. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 855, 176–180 (2000).
Datta, S., Siwek, D. F., Patterson, E. H. & Cipolloni, P. B. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse 30, 409–423 (1998).
Steriade, M., Datta, S., Paré, D., Oakson, G. & Curró Dossi, R. C. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J. Neurosci. 10, 2541–2559 (1990).
Moriizumi, T., Nakamura, Y., Tokuno, H., Kudo, M. & Kitao, Y. Synaptic organization of the pedunculopontine tegmental nucleus of the cat. Brain Res. 478, 315–325 (1989).
Datta, S. Cellular and chemical neuroscience of mammalian sleep. Sleep Med. 11, 431–440 (2010).
Soltani, S. et al. Sleep–wake cycle in young and older mice. Front. Syst. Neurosci. 13, 51 (2019).
Ulloor, J. & Datta, S. Spatio-temporal activation of cyclic AMP response element-binding protein, activity-regulated cytoskeletal-associated protein and brain-derived nerve growth factor: a mechanism for pontine-wave generator activation-dependent two-way active-avoidance memory processing in the rat. J. Neurochem. 95, 418–428 (2005).
Paxinos, G., Petrides, M., Huang, X. F. & Toga, A. W. The Rhesus Monkey Brain in Stereotaxic Coordinates (Elsevier Science, 2008).
Ramirez-Villegas, J. F., Willeke, K. F., Logothetis, N. K. & Besserve, M. Dissecting the synapse- and frequency-dependent network mechanisms of in vivo hippocampal sharp wave-ripples. Neuron 100, 1224–1240 (2018).
Leonard, T. K., Mikkila, J. M., Eskandar, E. N., Gerrard, J. L., Kaping, D., Patel, S. R., Womelsdorf, T., & Hoffman, K. L. Sharp wave ripples during visual exploration in the primate hippocampus. J. Neurosci. 35, 14771–14782 (2015).
Leppä, M. et al. Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage 31, 661–669 (2006).
Talbot, J. D. et al. Multiple representations of pain in human cerebral cortex. Science 251, 1355–1358 (1991).
Jones, A. K. et al. In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neurosci. Lett. 126, 25–28 (1991).
Grønlykke, L. et al. Remifentanil-induced spike activity as a diagnostic tool in epilepsy surgery. Acta Neurol. Scand. 117, 90–93 (2008).
Iannetti, G. D. et al. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc. Natl Acad. Sci. USA 102, 18195–18200 (2005).
Wise, R. G. et al. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage 16, 999–1014 (2002).
Goense, J. B. M. & Logothetis, N. K. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr. Biol. 18, 631–640 (2008).
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001).
Mortazavi, S., Thompson, J., Baghdoyan, H. A. & Lydic, R. Fentanyl and morphine, but not remifentanil, inhibit acetylcholine release in pontine regions modulating arousal. Anesthesiology 90, 1070–1077 (1999).
Lozito, R. J., La Marca, S., Dunn, R. W. & Jerussi, T. P. Single versus multiple infusions of fentanyl analogues in a rat EEG model. Life Sciences 55, 1337–1342 (1994).
Egan, T. D., Minto, C. F., Hermann, D. J., Barr, J., Muir, K. T. & Shafer, S. L. Remifentanil versus alfentanil: comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology 84, 821–833 (1996).
Bonafide, C. P., Aucutt-Walter, N., Divittore, N., King, T., Bixler, E. O. & Cronin, A. J. Remifentanil inhibits rapid eye movement sleep but not the nocturnal melatonin surge in humans. Anesthesiology 108, 627–633 (2008).
Besserve, M., Lowe, S. C., Logothetis, N. K., Schölkopf, B. & Panzeri, S. Shifts of gamma phase across primary visual cortical sites reflect dynamic stimulus-modulated information transfer. PLoS Biol. 13, e1002257 (2015).
Brillinger, D. R. Estimation of 2nd-order intensities of a bivariate stationary point process. J. R. Stat. Soc. B 38, 60–66 (1976).
Saleem, K. S. & Logothetis, N. K. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain (Academic, 2006).
Blair, R. C., & Karniski, W. An alternative method for significance testing of waveform difference potentials. Psychophysiology 30, 518–524 (1993).
Acknowledgements
We thank O. Eschenko and M. Constantinou for providing feedback on earlier versions of this work, and J. Werner and M. Schnabel for technical support during the development of this study. This research was supported by the Max Planck Society.
Author information
Authors and Affiliations
Contributions
N.K.L. conceived and designed the research. J.F.R.-V., Y.M., M.B. and N.K.L. performed the research. M.B. and N.K.L. supervised the research. J.F.R.-V., M.B., H.C.E., A.O. and N.K.L. contributed new reagents and analytical tools. J.F.R.-V. and N.K.L. analysed the data. J.F.R.-V., M.B. and N.K.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Ripple-triggered functional MRI responses in the macaque brain.
a, SWR-Triggered Peri-Event time courses. Typically cortical and subcortical brain regions are up and down modulated respectively, at the time of ripple-occurrence. b, Observed was a consistent (in every single experimental session) and robust down-modulation of Pons, LGN-Pulvinar (Pul) and the foveal and perifoveal regions of the primary visual cortex (V1). c, Average time courses of every brain regions of interest (ROI) in the peri-ripple epochs. d, Mean ripple-triggered fMRI responses in Pons, LGN-Pul and striate cortex, calculated based on the multiple regression (General Linear Model, that is, GLM) method selecting voxels by fitting a linear combination of two response models of different start points by 4 s. Trials with positive or negative BOLD responses (PBR or NBR) were selected with GLM only by for pons (Pontine Region and Parabrachial Nucleus). The selected PBR- and NBR trials were used to plot the responses in LGN and Striate Cortex. Left most plot shows the overall response in pons, that is, the average of all trials, just as it is shown in c. Middle and right most plots show the average response of the three structures for PBR- and NBR-Trials respectively. Shaded areas indicate SEM. Abbreviations: LGN Lateral Geniculate Nucleus, HP Hippocampus, PontReg Pontine Region Including Parabrachial Nucleus, fV1 – pfV1 Foveal, parafoveal and perifoveal striate cortex (V1).
Extended Data Fig. 2 Detailed description of the collected data.
The collected data (total of over 100 h of recordings) comprised a total of 630 10-min Data-Acquisition-Epochs, grouped in 66 groups, recorded during 42 experimental sessions (note that one session corresponds to one recording day). Experimental sessions consisted of concurrent functional MRI and electrophysiology recordings of spontaneous activity in 2 structures (24 sessions for PBn-HC, 6 sessions for PBn-LGN, 4 sessions for HC-LGN; animals A13, F12, K13, CM029, and E10), one session with the three structures (PBn-HC-LGN; animal K13) and 7 additional sessions with 3 structures including the LGN, VCX and hippocampus (LGN-HC-VCX; animals I09 and A13). A collation of the LFP recording sites for the PBn (25 exemplary experimental sessions; animals K13, CM029 and F12) and HC (26 exemplary experimental sessions; animals A13, E10, F12, I09 and K13) is reported here and in Extended Data Fig. 3. Electrode locations were derived from high-resolution anatomical scans, overlaid onto the corresponding coronal slices of the Paxinos rhesus monkey brain atlas or the Saleem–Logothetis combined histology-MRI of the rhesus monkey brain atlas. The remaining experimental sessions shared similar electrode positions to the ones reported here and in Extended Data Fig. 3, in consensus with the anatomical scans and the electrical profiles of the recorded signals.
Extended Data Fig. 3 Collation of the LFP recording sites in or around the parabrachial nucleus, from 25 exemplary recording sessions made in three monkeys (k13: 19 sessions; cm029: 4 sessions; f12: 3 sessions).
The location of the recording sites was derived from high-resolution anatomical scans and transposed onto the corresponding location on drawing of coronal slices of the rhesus macaque monkey brain. With the exception of k13m1, all recording sites were located inside the parabrachial nucleus. Relevant abbreviations: KF: Kolliker-Fuse; LPB, lateral parabrachial; LPBC, lateral parabrachial central; LPBD, lateral parabrachial dorsal; LPBE, lateral parabrachial external; LPBV, lateral parabrachial ventral; MPB, medial parabrachial; MPBE, medial parabrachial external; scp, superior cerebellar peduncle. The values in mm indicate the interaural distance of the atlas. All anatomical images have been adapted with permission from the Paxinos et al. rhesus monkey brain atlas45.
Extended Data Fig. 4 Collation of the LFP recording sites in the hippocampal formation.
The images display a total of 26 recording sessions made in 5 monkeys (A13, 3 sessions; E10, 1 session; F12, 3 sessions; I09, 1 session; K13, 18 sessions). The location of the recording sites was derived from high-resolution anatomical scans and transposed onto the corresponding location on drawing of coronal slices of the Saleem–Logothetis rhesus monkey brain atlas62,63 (adapted with permission). All recording sites were located inside the hippocampal formation, with 12 sessions made in CA1, 9 in both CA1 and CA2, and 5 in CA3 or CA4. Relevant abbreviations: CA1-4, Ammon’s Horn 1-4; DG, dentate gyrus. Optic Tract at +18 LGN starts (clear in MRI) = +10 session AP(mm) Possible HP regions.
Extended Data Fig. 5 Time and frequency profiles of reported hTheta oscillations and PGOw.
a, Exemplary traces of hTheta episodes (left) and their associated spectra (right) reported in various macaque studies7,22,47. b, Same as a, but for the pontine and geniculate components of PGO events in independent feline and non-human primate studies14,40. Spectra are based on autoregressive power spectral density estimate, using the Yule-Walker method with order 6, using MATLAB’s function pyulear).
Extended Data Fig. 6 Pontine events characteristics in the ponto-geniculo-hippocampal recordings.
a, Pontine peri-event LFP activity for p-waves (uncoupled, black trace), ripple-coupled (red trace) and theta-coupled (blue trace) PGO waves: averaged time courses (top) and spectrogram averages (bottom). Shaded areas indicate SEM. b, Analogous to panel a, but for LGN activity. c, Peri-event BLP signals averages in the 4–10 Hz LFP range in PBn, LGN and hippocampus. Whereas PGO waves occur in all structures, P-waves display significant power increases only in the pons (P < 0.01, permutation t-test for PBn responses; P > 0.05, permutation t-test for LGN and hippocampal responses). Shaded areas indicate SEM. d, Consistency score for the detection of pontine waves (P-waves and PGO waves) using the recording pairs PBn-LGN and PBn-HC (Methods). Statistics are computed across experiments of the three-structure experimental session. e, Exemplary LFP traces of ongoing LGN, neocortical, that is, primary VCX and HC activity. The dashed line encloses a PGO event. f, Expanded version of the event highlighted in e. g, Statistics of the phase shift (left) and time lag (right) between LGN and visual cortex during PGO events across experimental sessions. Individual dots (right subpanel) correspond to each of the 7 experimental sessions where the triad LGN-VCX-HC were recorded, animals A13, D11 and i09. h, Putative causal interaction between LGN and VCX assessed using transfer entropy (TE) (P > 0.2890 sign test; N = 7 experimental sessions, animals A13, D11 and i09). i, Point-process conditional intensity functions between these events and detected PGO waves in LGN-VCX-HC sessions (asterisks indicate significant coupling, P < 0.01 permutation test against an H0 distribution of random point proceses of the same rate; 7 experimental sessions; animals A13, D11 and i09). For the visual cortex data, we detected candidate PGO waves as peaks of low-frequency (2–15 Hz) LFP power exceeding an event-related threshold proportional to the standard deviation of the signal (z-scores) in LGN. Theta (2-15 Hz), gamma (25-75 Hz) and ripples (90-190 Hz) were detected independently from the hippocampal LFP traces (N = 7 experimental sessions; animals A13, D11 and I09). Point-process cross-correlation analysis revealed that PGO-associated potentials in LGN significantly co-occur with hippocampal theta and SWR, but not gamma episodes (Panel i). Analogous to our analysis of PBn-LGN PGO waves, albeit more variable, we found a phase lag of visual cortex activity with respect to LGN activity in the PGO frequency band (2-15 Hz) (Panel g). This phase lag was consistent across all sessions and animals where LGN and visual cortex activities were recorded (mean phase lag with 95% circular confidence interval 8.76 ± 8.44 degrees; P < 10−2 circular test for the significance of the median; N = 7 experimental sessions, animals A13, D11 and I09). The corresponding time delay between LGN and VCX found in this analysis is consistent with previous studies in cats26. We found difficult to detect a putative causal influence of the phase of LGN on the phase of visual cortex during peri-PGO potentials, as hinted by the non-significant differences in the LGN-to-VCX interaction direction as compared to the opposite VCX-to-LGN direction (Panel h; P = 0.2890, sign test for the comparison between TE values associated with the LGN-to-VCX direction and the VCX-to-LGN direction; N = 7 experimental sessions, animals A13, D11 and I09). The results of this causal inference analysis for PBn-LGN and LGN-VCX experimental sessions, were found consistent with the spectral independence-based causal inference analysis introduced in the next subsection of the manuscript in the context of PBn-hippocampus PGO event-related interactions (Supplementary Methods). These results indicated that causal inference might be difficult to detect in LGN-VCX recording pairs due to the highly recurrent connectivity of the macaque visual cortex. In addition, our results indicate that the detected phasic events in our recordings are indeed potentials that co-occur in the brainstem, thalamic nuclei, and neocortex, thus corresponding to PGO waves11.
Extended Data Fig. 7 Hippocampal event features in PBn-HC recordings of macaque monkeys.
a, Detected SWR (red arrows) and theta-like events in an exemplary hippocampal raw LFP trace (0.5-190 Hz) and its filtered version in the ripple frequency band (90-190 Hz). SWR are clearly marked by the occurrence of high-frequency oscillations as shown in the top insets. b, Theta- (left panel), gamma- (middle panel) and ripple-triggered (right panel) complex Morlet-wavelet power spectrograms. c, Averaged zero-lag spectral profiles in correspondence with each hippocampal event type. Shaded areas indicate SEM. d, Bivariate distribution of FWHM versus frequency peak (after Logothetis et al., 2012) (left panel). e, f, Frequency peak (e) and FWHM (f) box plots are also shown separately. Colours indicate the hippocampal event type shown in B. On each box, the top and bottom are the 25th and 75th percentiles of the samples, respectively; the middle dot of each box is the sample median; the dashed lines extending below and above each box are drawn to the extreme points; black crosses in the diagrams are the outliers of the samples. g, Theta- (top), gamma- (middle) and SWR-triggered (bottom) BLP signals population grand averages in and beyond the LFP frequency range: delta (1–4 Hz), theta (4–10 Hz), spindle (10–16 Hz), sigma (16–30 Hz), slow and high gamma (30–70 Hz; 70–90 Hz, respectively), ripple (90–190 Hz) and high-frequency oscillations (190–320 Hz) and MUA (801–2500 Hz). BLP signals were z-scored and averaged across all recording tips. All responses were thresholded for a significant deviation from zero at a statistical significance level P < 0.05 (permutation test) and Bonferroni-corrected across frequency bands. h, Time course population averages of BLP modulations in hippocampus over MUA (801–2500 Hz) for SWR (top), gamma (middle) and theta (bottom) episodes. Note the prevalent negative modulation associated with theta events, as opposed by a consistent increase of activity associated with ripple events. i, Point-process conditional intensities (cross-correlograms) accounting for the relationship between the occurrences of theta, gamma and SWR episodes. Conditional intensities were computed from pooled experiments across pons-hippocampus experimental sessions, at a resolution of 50 ms bins. Asterisks indicate a significant relationship (P < 0.01, Bonferroni-corrected) at and around the time of the occurrence of theta (left), gamma (middle) and SWR (right) events, according to an H0 distribution derived from cross-correlograms of randomized point processes of the same rate. The reference event for computing the conditional intensity is indicated by the first event of each pair in the top part of each plot. For panels b–i, N = 24 experimental sessions contributed to the statistics; animals CM031, F12 and K13; virtually identical event profiles were obtained with sessions from animals A13, D11, i09 and e10).
Extended Data Fig. 8 PGO wave oscillation and discharge statistics across all experimental sessions and exemplary time courses.
a, Box plots illustrating the proportion of PGO-waves in each experimental session. The session marked in red (bottom subpanel) corresponds to the session in which PBn, LGN and hippocampus were recorded simultaneously (N = 29 experimental sessions; animals CM029, K13, F12, CM031; sessions with animals E10, A13, D11 and i09 produced virtually identical results). b, Box plots displaying the pooled statistic of the proportion of PGO waves (right) across all experimental sessions. About 30% of all pontine events are classified as PGO waves (N = 29 experimental sessions; animals CM029, K13, F12, CM031). c, Two-dimensional projection of type I and type II PGO waves (red and blue LFP traces, respectively) using Laplacian eigenmaps for one exemplary experimental session. The projection is computed using the peri-event MUA (801-2500 Hz). In this case, type I PGO waves are segregated to the left, whereas type II PGO waves are segregated to the right. d, Zoomed-in version of two patches enclosed by a dashed square in c. Type I PGO waves usually display one biphasic cycle, whereas type II PGO waves display more oscillatory behaviour. e, PGO-wave time course averages for the experimental session in c and d. f, Global (across-sessions) statistics of the number of oscillatory cycles displayed by PGO waves recorded in PBn (N = 24 experimental sessions, animals K13, F12 and CM031). The empirical distribution associated with type II PGO waves is consistently shifted to the right, indicating a larger number of oscillations. g, Analogous to f, but for SWR and theta events recorded in hippocampus. h, Peri-event time histogram of pontine multi-unit discharges (25-ms bins) during the occurrence of type I (left) and type II (centre) PGO waves, and P-waves (right). Shaded areas indicate SEM across experimental sessions. i, Multi-unit modulation associated with P-waves and PGO-wave subtypes. Note the stronger multi-unit modulation associated with type I and type II PGO waves. For panels h, i, N = 24 experimental sessions, K13, F12 and CM031.
Extended Data Fig. 9 PGOw in LGN.
a, Two-dimensional projection of type I and type II PGOw (red and blue LFP traces, respectively) using Laplacian eigenmaps for one exemplary PBn-LGN experimental session. Each trace corresponds to one event. Events are classified on the basis of their peri-event MUA (801–2500 Hz), also used to compute the projection. Note the segregation of type I PGOw and type II PGOw in this space. b, Zoomed-in version of two patches enclosed by a dashed square in a. Type I PGOw usually display one biphasic cycle, whereas type II PGOw display more oscillatory behaviour. c–e, PGO-wave LFP (c) spectrogram (d) and MUA (801–2,500 Hz) (e) averages are shown for the selected events of this experimental session exhibited in a and b. f, g, SDR results for putative PBn-LGN and LGN-VCX causal interactions during PGOw. Unlike that of neocortex, note the significantly increased SDR in the PBn → LGN interaction direction, indicating a putative causal effect of PBn over LGN PGO wave activity. Here we asked whether the brainstem exerted a causal influence on LGN, and whether LGN exerted a similar influence on VCX activity during the occurrence of PGOw. SDR analysis confirmed that PGOw propagate from PBn to LGN (P < 0.004 sign test, N = 7 experimental sessions; Panel f). In addition, we did not find statistically significant evidence for the direction of causation for the case of LGN and VCX recording pairs (P = 1 sign test, N = 7 experimental sessions; Panel g).
Extended Data Fig. 10 Multiunit activity (MUA) and ripple- and theta-event density functions (EDF) for the sessions with recordings from two PGO-related structures.
The events included in this figure were selected as “propagating”, namely as events in the first PGO-related structure, which induced a suprathreshold response in a second structure. The sign of the Phase-Locking Value (PLV) implying the that second structure is following the first did not change the profile of responses shown here. a, Pontine MUA and Hippocampal Event Density Functions (EDFs) for the single PBn-LGN-HC session (left group of plots), and for LGN-VCx-HC sessions, where LGN was the first and Cortex the second PGO-related anatomical structure (right group of plots). Experimental sessions and groups associated with each group of plots are indicated at the top. Type-I PGO events, as shown in main text figures, are strongly associated with occurrence of hippocampal ripples, while the Type-II PGO events are associated with theta-bouts. Shaded areas indicate SEM. b, Same as a, but in this case events were simply detected in the first PGO-associated structure and were subsequently clustered in an unsupervised fashion using the Growing Neural Gas-based clustering algorithm (Supplementary Methods). Grey areas correspond to SEM estimated from the data of all groups (spont & spont1) across experimental sessions. c, Percentage of propagating events (PGO waves) that were predicted by unsupervised clustering, assuming the detection of PGO waves using a second PGO-associated structure as the ground truth (left subpanel). For most sessions, the events are consistently predicted as PGO waves, corresponding to more than 80% of the ground-truth events. The percentage of each PGO wave type (right subpanel) shows that there are characteristic differences and variability across sessions, probably reflecting the state of the animal during individual preparations. Sessions in which hippocampus and LGN were recorded (animal e10) yielded virtually identical results.
Extended Data Fig. 11 Pontine MUA and hippocampal EDF profiles for the largest session-groups used in the current report.
a, PBn MUA profiles for type I, type II PGO waves, that is, associated with hippocampal SWR and theta waves, and P-waves (uncoupled events). b, EDFs associated with hippocampal SWR. Note the increased SWR density, illustrating that SWR notably co-occur with type I PGO waves. c, Same as b, but for theta events. Recordings from PBn only did not permit the exact identification of propagating PGO-events. However, as mentioned in the previous figures, strongly increasing all thresholds used for clustering the responses yielded virtually the same results.
Extended Data Fig. 12 Brain state scoring on the basis hippocampal and neocortical recordings.
a, Exemplary broad-band (0.5-200 Hz) (top) LFP trace from the hippocampal CA1, and its filtered version in the ripple frequency band (90-190 Hz). NREM (red shades) and REM (blue shades) episodes have been scored on the basis of power-power correlations between two recording sites located close by (150 μm) in the same hippocampal subfield. Similar scoring can be done using neocortical recordings, on the basis of the LFP low-frequency power (<12 Hz, Supplementary Methods). Note the occurrence of SWR episodes (increases in high-frequency oscillations) during epochs classified as NREM-like states, and absence thereof during epochs classified as REM-like states. b, State-scoring consistency for experimental sessions in which hippocampus and neocortex were recorded (left), which was found to correlate with ripple signal-to-noise ratio (right; here measured in SD, computed from surrogate epochs were no ripples are observed) (statistics are derived from groups spont & spont1; N = 7 experimental sessions, animals A13, D11 and i09). c, Exemplary LFP traces displaying neocortical delta waves (1-4 Hz) oscillations, a well-known landmark of desynchronized (NREM) sleep. d, State-associated LFP power across neocortical, LGN and hippocampal recording sites in the delta [1-4 Hz] and theta [5-12 Hz] (P < 0.001 Wilcoxon’s ranksum test for the difference of LFP power across states, Bonferroni-corrected; N = 7 experimental sessions, animals A13, D11 and i09). e, Proportion of all SWR and theta episodes across sleep-like states, and across the two different state-scoring procedures (P > 0.05 pair-wise Wilcoxon’s ranksum test, uncorrected; N = 7 experimental sessions, animals A13, D11 and i09). f, Same as a, for one experimental session in which no neocortical activities were recorded. The scoring strongly mirrors the one performed when neocortical recordings are available. The observed changes in event occurrence (coloured dots at the top of the panel) are mirrored by fluctuations the z-scored multi-unit activity (MUA) (801-2500 Hz) of the mEFP corresponding to the same recording site illustrated in a. NREM states are accompanied by strong up-down state-like modulations (bottom subpanel), and MUA episodes, essentially absent during REM states. g, Averaged power-power correlations between two recording sites located close by (150 μm) in the same hippocampal subfield for NREM (left) and REM (right) episodes, for: one exemplary LGN-CTX-HC experimental session (top); the only PBN-LGN-HC experimental session (middle); and across all 24 PBN-HC experimental sessions (bottom). Note the increased high-frequency (>100 Hz) coupling associated with SWR episodes during the NREM-like state, which is otherwise absent during the REM-like state (black and white arrows).
Extended Data Fig. 13 Hippocampus-PBn field coherency, and hippocampal multi-unit spiking activity, as modulated by brainstem PGO waves.
a, Coherency maps grand-averages of PGO-uncoupled (left) and PGO-coupled (right) hippocampal SWR (top) and theta episodes (bottom). b, Zero-lag profiles of the events in a. c, PBn-LFP-triggered modulation of hippocampal multi-unit discharges for PGO-uncoupled (left) and PGO-coupled (right) SWR (red) and theta (blue) events. Shaded areas indicate SEM. d, Depth of hippocampal multi-unit modulation with respect to PBn LFP during the occurrence of PGO-uncoupled (dashed boxes) and PGO-coupled (continuous boxes) SWR (red) and theta episodes (blue). Note the significantly greater multi-unit modulation depth of PGO-coupled events as compared to that of PGO-uncoupled events (P < 10−5 Wilcoxon’s ranksum test, Bonferroni-corrected). e, Circular histograms of the PBn LFP (2–15 Hz) phase at maximal hippocampal multi-unit spike discharge for PGO-coupled SWR (red) and theta (blue) events. Panels a–e were derived from N = 24 experimental sessions; animals K13, F12 and CM31.
Extended Data Fig. 14 Posture of the animal during data collection.
a, Site-Localization. The chambers for deep brain recordings were designed to be positioned at an optimum angle to prevent possible injuries to large blood vessels. In more than 30 reversible experiments with electrodes under anaesthesia, the animals – with few exceptions – did not show any complications as a result of the electrode placement in the pons region. Figure: Example design of a pons chamber using MR angiography. The Brainsight system (Rogue Research, https://www.rogue-research.com/) was used for the precise implantation of electrophysiology chambers aimed to explore deep brain structures (for example, the lateral geniculate nucleus, and the parabrachial nucleus). Before the surgery, anatomical scans of the animal’s head were produced with markers to obtain data on the skull shape and pertinent target structures for tailor-made implants. The brain-vasculature was reconstructed, and a path with lowest probability of hitting large vessels/arteries was chosen. During the surgery, the position of the implant was determined by the Brainsight computer programme, which can superimpose a virtual implant with its trajectory and targets on the reconstructed skull using the pointer tool. These techniques can prevent the undesirable misplacement of recording chambers, which could be the case when there is a small difference in shape between the skull and the implant (for example, an angle of 5 degrees causes a shift of about 2.6 mm under a 30-mm chamber. This shift would be sufficient to miss a smaller deep brain structure). At right of panel a, is an example of multisite/multilaminar electrode in two magnifications. b, Placement of the electrodes before the combined physiology-fMRI experiments. c, It shows the posture of the animal during the experiment. Due to the fact that all magnets were vertical for reducing any stress to the animal in alert monkey experiments, the anaesthetized monkeys were prepared with all measure for maintaining normal blood pressure (foot-bandage), and temperature, as well as for measuring of physiological parameters. During the maintenance of aneesthesia, as well as data collection, the animal was always in a “sitting” position with closed eyes with all necessary items for the maintenance of anaesthesia and measurement of physiological parameters. d, The animal before shifting the chair into the magnet.
Supplementary information
Rights and permissions
About this article
Cite this article
Ramirez-Villegas, J.F., Besserve, M., Murayama, Y. et al. Coupling of hippocampal theta and ripples with pontogeniculooccipital waves. Nature 589, 96–102 (2021). https://doi.org/10.1038/s41586-020-2914-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2914-4
This article is cited by
-
Overnight neuronal plasticity and adaptation to emotional distress
Nature Reviews Neuroscience (2024)
-
A medullary hub for controlling REM sleep and pontine waves
Nature Communications (2023)
-
Prefrontal cortical regulation of REM sleep
Nature Neuroscience (2023)
-
Neurocognitive, mood changes, and sleepiness in patients with REM-predominant obstructive sleep apnea
Sleep and Breathing (2023)
-
REM sleep is associated with distinct global cortical dynamics and controlled by occipital cortex
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.