Abstract
Infant cries evoke powerful responses in parents1,2,3,4. Whether parental animals are intrinsically sensitive to neonatal vocalizations, or instead learn about vocal cues for parenting responses is unclear. In mice, pup-naive virgin females do not recognize the meaning of pup distress calls, but retrieve isolated pups to the nest after having been co-housed with a mother and litter5,6,7,8,9. Distress calls are variable, and require co-caring virgin mice to generalize across calls for reliable retrieval10,11. Here we show that the onset of maternal behaviour in mice results from interactions between intrinsic mechanisms and experience-dependent plasticity in the auditory cortex. In maternal females, calls with inter-syllable intervals (ISIs) from 75 to 375 milliseconds elicited pup retrieval, and cortical responses were generalized across these ISIs. By contrast, naive virgins were neuronally and behaviourally sensitized to the most common (‘prototypical’) ISIs. Inhibitory and excitatory neural responses were initially mismatched in the cortex of naive mice, with untuned inhibition and overly narrow excitation. During co-housing experiments, excitatory responses broadened to represent a wider range of ISIs, whereas inhibitory tuning sharpened to form a perceptual boundary. We presented synthetic calls during co-housing and observed that neurobehavioural responses adjusted to match these statistics, a process that required cortical activity and the hypothalamic oxytocin system. Neuroplastic mechanisms therefore build on an intrinsic sensitivity in the mouse auditory cortex, and enable rapid plasticity for reliable parenting behaviour.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are further available at figshare (https://www.nih.figshare.com) using https://doi.org/10.6084/m9.figshare.c.5043830, and from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Source code is available at figshare (https://www.nih.figshare.com) using https://doi.org/10.6084/m9.figshare.c.5043830.
Change history
06 November 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41586-020-2898-0
References
Swain, J. E., Kim, P. & Ho, S. S. Neuroendocrinology of parental response to baby-cry. J. Neuroendocrinol. 23, 1036–1041 (2011).
Lingle, S., Wyman, M. T., Kotrba, R., Teichroeb, L. J. & Romanow, C. A. What makes a cry a cry? A review of infant distress vocalizations. Curr. Zool. 58, 698–726 (2012).
Dulac, C., O’Connell, L. A. & Wu, Z. Neural control of maternal and paternal behaviors. Science 345, 765–770 (2014).
Zeskind, P. S. in Infant Crying: Theoretical and Research Perspectives (eds Boukydis, C. F. Z. and Lester, B. M.) (Springer, New York, 1985).
Ehret, G., Koch, M., Haack, B. & Markl, H. Sex and parental experience determine the onset of an instinctive behavior in mice. Naturwissenschaften 74, 47 (1987).
Koch, M. & Ehret, G. Estradiol and parental experience, but not prolactin are necessary for ultrasound recognition and pup-retrieving in the mouse. Physiol. Behav. 45, 771–776 (1989).
Elyada, Y. M. & Mizrahi, A. Becoming a mother-circuit plasticity underlying maternal behavior. Curr. Opin. Neurobiol. 35, 49–56 (2015).
Marlin, B. J., Mitre, M., D’amour, J. A., Chao, M. V. & Froemke, R. C. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504 (2015).
Noirot, E. in Advances in the Study of Behavior: IV (eds Lehrman, D. S. et al.) (Academic Press, New York, 1972).
Ehret, G. Infant rodent ultrasounds — a gate to the understanding of sound communication. Behav. Genet. 35, 19–29 (2005).
Liu, R. C., Miller, K. D., Merzenich, M. M. & Schreiner, C. E. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J. Acoust. Soc. Am. 114, 3412–3422 (2003).
Lindová, J., Špinka, M. & Nováková, L. Decoding of baby calls: Can adult humans identify the eliciting situation from emotional vocalizations of preverbal infants? PLoS One 10, e0124317 (2015).
Weatherholtz, K. & Jaeger, T. F. Speech perception and generalization across talkers and accents. Oxford Research Encyclopedias https://doi.org/10.1093/acrefore/9780199384655.013.95 (2016).
Holt, L. L. & Lotto, A. J. Speech perception as categorization. Atten. Percept. Psychophys. 72, 1218–1227 (2010).
Petkov, C. I. & Jarvis, E. D. Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front. Evol. Neurosci. 4, 12 (2012).
Castellucci, G. A., Calbick, D. & McCormick, D. The temporal organization of mouse ultrasonic vocalizations. PLoS One 13, e0199929 (2018).
Ehret, G. & Bernecker, C. Low-frequency sound communication by mouse pups (Mus musculus): wriggling calls release maternal behavior. Anim. Behav. 34, 821–830 (1986).
Uematsu, A. et al. Maternal approaches to pup ultrasonic vocalizations produced by a nanocrystalline silicon thermo-acoustic emitter. Brain Res. 1163, 91–99 (2007).
Gaub, S. & Ehret, G. Grouping in auditory temporal perception and vocal production is mutually adapted: the case of wriggling calls of mice. J. Comp. Physiol. 191, 1131–1135 (2005).
Kuchibhotla, K. V. et al. Parallel processing by cortical inhibition enables context-dependent behavior. Nat. Neurosci. 20, 62–71 (2017).
Liu, R. C., Linden, J. F. & Schreiner, C. E. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur. J. Neurosci. 23, 3087–3097 (2006).
Metherate, R. & Ashe, J. H. Facilitation of an NMDA receptor-mediated EPSP by paired-pulse stimulation in rat neocortex via depression of GABAergic IPSPs. J. Physiol. (Lond.) 481, 331–348 (1994).
Jean-Richard-Dit-Bressel, P., Killcross, S. & McNally, G. P. Behavioral and neurobiological mechanisms of punishment: implications for psychiatric disorders. Neuropsychopharmacology 43, 1639–1650 (2018).
Butts, D. A. & Goldman, M. S. Tuning curves, neuronal variability, and sensory coding. PLoS Biol. 4, e92 (2006).
Katlowitz, K. A., Picardo, M. A. & Long, M. A. Stable sequential activity underlying the maintenance of a precisely executed skilled behavior. Neuron 98, 1133–1140.e3 (2018).
Valtcheva, S. & Froemke, R. C. Neuromodulation of maternal circuits by oxytocin. Cell Tissue Res. 375, 57–68 (2019).
Mitre, M. et al. A distributed network for social cognition enriched for oxytocin receptors. J. Neurosci. 36, 2517–2535 (2016).
Pekarek, B. T., Hunt, P. J. & Arenkiel, B. R. Oxytocin and sensory network plasticity. Front. Neurosci. 14, 30 (2020).
Zador, A. M. A critique of pure learning and what artificial neural networks can learn from animal brains. Nat. Commun. 10, 3770 (2019).
Dimidschstein, J. et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat. Neurosci. 19, 1743–1749 (2016).
Kerlin, A. M., Andermann, M. L., Berezovskii, V. K. & Reid, R. C. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67, 858–871 (2010).
Tasaka, G. I. et al. The temporal association cortex plays a key role in auditory-driven maternal plasticity. Neuron 107, 566–579.e7 (2020).
Acknowledgements
We thank I. Carcea, C. L. Ebbesen, W. Gan, E. Glennon, M. Insanally, K. A. Katlowitz, K. Kuchibhotla, D. Lin, M. A. Long, N. López Caraballo, B. J. Marlin, R. Oyama and J. A. Schiavo for comments, discussions and technical assistance. The AAV.mDLX.GcAMP6f virus (Fig. 2a) was a gift from J. Dimidschstein and G. Fishell. S. E. Ross created artwork in Figs. 1b, d, 3a, 4a, Extended Data Fig. 2c. We thank K. Furman and M. Hopkins for their help in developing the operant paradigm used in Fig. 1h, i. This work was funded by an NSF Graduate Research Fellowship (J.K.S. and K.A.M.); a Leon Levy Foundation Postdoctoral Fellowship and Brain & Behavior Research Foundation NARSAD Young Investigator Award (S.V.), as well as a Program Projects Grant (NS074972), the BRAIN Initiative (NS107616), NICHD (HD088411), NIDCD (DC12557), a McKnight Scholarship, a Pew Scholarship, and a Howard Hughes Medical Institute Faculty Scholarship (R.C.F.).
Author information
Authors and Affiliations
Contributions
J.K.S. conducted behavioural studies, in vivo optogenetics and two-photon calcium imaging. S.V. performed in vivo whole-cell recordings. S.C.S. and C.J.B.-M. performed in vitro whole-cell recordings. K.A.M. wrote the code and made the hardware for operant testing. In vivo imaging and whole-cell data were analysed by J.K.S. In vitro whole-cell data were analysed by J.K.S., S.C.S. and C.J.B.-M. J.K.S. and R.C.F. designed the study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Maria Neimark Geffen, Daniel Polley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
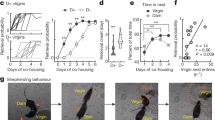
Extended Data Fig. 1 Stimulus library of prototypical and morphed pup calls.
a, A set of six prototypical pup calls were selected from a library of pre-recorded calls. Prototypical calls had 4–5 syllables and an average ISI of 150–200 ms (bin: 175 ± 25 ms). Total duration of each prototype was approximately 1 s. b, Example of one prototypical call morphed in the temporal domain. Time was added or subtracted from the ISI to slow down or speed up the calls, respectively. Other features (for example, frequency content) remained the same across all temporal morphs. A set of seven morphs was generated for each prototypical call (bin centre: 75, 125, 225, 275, 375, 575 and 975 ms; bin size: ± 25 ms), resulting in a library of 42 pup call sounds. Colour indicates overall speed or duration of ISIs, with red representing faster calls with shorter ISIs, and blue representing slower calls with longer ISIs.
Extended Data Fig. 2 Pup call ISIs drive retrieval and approach behaviour in experienced females.
a, Retrieval in cold pup assay from Fig. 1c across dams and experienced virgins. Dams and experienced virgins did not differ in their retrieval behaviour. Warm pups: dams (85.2%, n = 27 trials) vs EVs (75.0%, n = 28), P = 0.50; prototypes (‘proto’): dams (91.7%, n = 12) vs. EVs (66.7%, n = 18), P = 0.19; cold pups: dams (25.0%, n = 12) vs EVs (38.5%, n = 13), P = 0.67 (two-tailed Fisher’s test). Retrieval rate ± 95% binomial confidence intervals. b, Latency to retrieve on successful retrieval trials from Fig. 1c. Latencies were binned based on whether ISIs elicited retrieval at similar rates to warm pups. Latencies to retrieve cold pups and cold pups dubbed over with slow morphs (single syllables (‘SS’) and 575-ms ISIs; n = 17 trials) were longer than for warm pups (n = 44; P = 0.02) or cold pups dubbed over with morphs containing ISIs between 75 and 375 ms (n = 82; P = 0.001) (Kruskal–Wallis H-test with Dunn’s correction). Median ± interquartile range. c, A y-maze was used to assess approach towards speakers playing pup calls versus temporal morphs in the absence of a live pup. A speaker in one room played a prototypical pup call, while a competing speaker in the other room played its spectrally-matched, temporal morph. Mice were given two minutes to enter a room. Data from dams and experienced virgins were pooled. d, When competing morphs contained ISIs between 25 and 149 ms (62.5 ms: 44.2%, n = 52 total trials) or between 201 and 350 ms (275 ms: 55.6%, n = 45), experienced females showed no significant preference between the prototype and morph (compared to chance (0.50) using two-tailed binomial test; 62.5 ms, P = 0.49; 275 ms, P = 0.46). Mice showed a significant preference for approaching prototypes when ISIs were slower than 350 ms; 425 ms (25.0%, n = 20), P = 0.04; 575+ ms (15.8%, n = 19), P = 0.005. Data binned ± 75 ms except 575+. Retrieval rate ± 95% binomial confidence intervals. *P < 0.05, **P < 0.01.
Extended Data Fig. 3 Two-photon calcium imaging of auditory cortical responses to pup calls and pure tones.
a–c, Neuropil correction on example datasets (n = 1 region containing excitatory neurons; n = 1 region containing inhibitory neurons). a, Top, correction was performed by measuring background fluorescence in the neuropil (‘NP’) surrounding each ROI (green) and local vasculature (‘V’). Bottom, example ∆F/F traces from an excitatory neuron before and after correction (Methods). b, c, Neuropil correction had no significant effect on prototype-evoked ∆F/F (%) in excitatory neurons (b, n = 52 neurons, P = 0.57) or inhibitory neurons (c, n = 64 neurons, P = 0.14; two-tailed unpaired Kolmogorov–Smirnov test). d, Example ∆F/F traces from three neurons acquired at 16 Hz; coloured number = ∆F/F (%). Responses from this experienced virgin were consistent with neuronal responses acquired at 4 Hz (Fig. 1e). e, Percentage of prototype-responsive excitatory neurons in experienced (n = 9 mice) and naive virgins (n = 12; P = 0.74; two-tailed unpaired t-test). f, Example heat maps of prototype-responsive neurons from an experienced (left) and naive virgin (right). g, h, Raw neuronal tuning from experienced (n = 9) and naive virgins (n= 12) summarized in Fig. 1f. i–m, Tuning to temporally-modulated tone sequences. i, Example stimulus set: five sequential tone pips (for example, 32 kHz, 80 ms) with the following ISIs: 75, 175, 375 or 575 ms (ISI bin ± 25 ms). j, Left, example ∆F/F traces evoked by temporally-modulated sequences of 32 kHz tones. Right, sample cell quantification. ∆F/F (%) normalized to the prototypical stimulus. k, Tuning width (normalized ∆F/F averaged across all stimuli). Experienced: n = 3 mice, n = 94 neurons; naive: n = 5, n = 45; P = 0.97 (unpaired two-tailed t-test). l, Sample imaging region from a naive virgin. Prototypical calls and temporally-modulated 32 kHz tones with ISIs approximately 175 ms (5.5 Hz) activated a subset of the same cells (green). These neurons could have distinct temporal tuning to ISIs (inset). m, We observed higher normalized ∆F/F values to temporally modulated tones (n = 5 mice) than to pup call morphs (n = 11–12) in naive auditory cortex (75 ms, P = 0.009; 375 ms, P = 0.04; 575 ms: P = 0.004; unpaired two-tailed Mann–Whitney test). Median ± interquartile range. All data are mean ± s.e.m. (except in m). *P < 0.05, **P < 0.01.
Extended Data Fig. 4 Temporal tuning to pup calls in left auditory cortex reflects behavioural-salience of ISIs and retrieval probability.
a–c, Excitatory neuronal tuning in left vs right auditory cortex of experienced virgins. a, Individual mouse tuning (right auditory cortex, n = 4 mice). b, Tuning normalized to prototypes in the left (n = 9 mice from Fig. 1f) and right (n = 4) auditory cortex of experienced virgins. Mean ± s.e.m. c, Tuning width (75–375 ms) in left vs right auditory cortex of mice in b (P = 0.003; unpaired two-tailed Mann–Whitney test). Median ± interquartile range. d, In experienced auditory cortex (n = 9 mice), evoked ∆F/F values were correlated with ISI probability (Pearson’s r = 0.86, P = 0.006; two-tailed). Colours reflect ISI bins reported in Fig. 1a. e, Qualitatively, we observed broad temporal tuning in the left auditory cortex of a lactating dam (n = 1 dam, n = 10 single-cell tuning curves). Mean ± s.e.m. f, Two example experienced virgins that exhibited unreliable retrieval behaviour on 10% (left) and 30% (right) of trials in a standard pup retrieval test. Temporal tuning at baseline (open circles) broadened following the onset of reliable retrieval behaviour (100% of trials, closed circles). ‘Days’ denote days of co-housing with a dam and litter. Left, day 0: n = 21 neurons, day 2: n = 26. Right, day 1: n = 57, day 2: n = 26. Mean ± s.e.m. g, Cumulative distribution of temporal tuning widths before (n = 2 mice, n = 78 neurons) and after the onset of reliable retrieval (n = 52). A larger proportion of neurons were more broadly tuned (higher normalized ∆F/F values) when experienced virgins retrieved on 100% of trials (P < 0.0001; two-tailed unpaired Kolmogorov–Smirnov test). *P < 0.05, **P < 0.01.
Extended Data Fig. 5 Pup-naive virgins did not increase the number of times they pressed a lever to turn off prototypes or morphs.
a, Normalized learning trajectories from all virgins tested in the instrumental paradigm from Fig. 1h, i (n = 7 mice per group). Each line denotes an individual virgin’s learning curve. b, The total number of levers presses in a session (which turns off continuously playing prototypes or morphs) did not increase by session 8, regardless of the stimulus group (n = 7 mice per group; 175 ms, P = 0.44; 75 ms, P = 0.19; 575 ms, P = 0.14; paired two-tailed t-test).
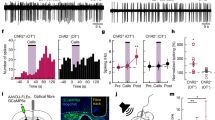
Extended Data Fig. 6 Experience-dependent neuronal and synaptic temporal tuning in auditory cortex.
a, Interneuron temporal tuning curves from experienced virgins (n = 5 mice). Each line denotes an individual mouse. b, As in a in naive virgins (n = 6). c, Tuning width across behaviourally salient ISIs 75–375 ms (average normalized ∆F/F). Excitatory neuronal tuning curves were significantly broader in experienced virgins (n = 9 mice) than in naive virgins (n = 12; P = 0.001). In naive cortex, interneuron temporal tuning was broader than excitatory tuning (NVinh: n = 6; P = 0.03). Interneuron tuning width did not differ between experienced (n = 5) and naive virgins (P > 0.99; one-way ANOVA, Bonferroni correction). Mean ± s.e.m. d, Slope of population tuning curves at the behavioural transition (375–975 ms). Slopes in experienced virgins were significantly negative. EVexc: n = 7 mice, P = 0.02; NVexc: n = 11, P = 0.84; EVinh: n = 5, P = 0.008; NVinh: n = 6, P = 0.24 (one-sample t-test to 0.0). Mean ± s.e.m. e, Excitatory synaptic tuning in experienced (left, n = 12 cells) and naive cortex (right, n = 14 cells). EV: all comparisons, P > 0.05. NV: 175 vs 125, P = 0.006; 175 vs 275, P = 0.003; 175 vs. 575, P = 0.02 (repeated measures one-way ANOVA, Bonferroni correction). f, Correlation of EPSCs with ISIs in experienced (left, n = 12 cells; Pearson’s r = −0.37, P = 0.009) and naive cortex (right, n = 14; Pearson’s r = −0.13, P = 0.35; two-tailed). g, Excitatory synaptic tuning in the auditory cortex of lactating dams (left, n = 4 cells) and experienced virgins (right, n = 8) (repeated measures one-way ANOVA, Bonferroni correction). h, Prototype and morph-evoked EPSCs did not differ between dams (n = 4 cells) and experienced virgins (n = 8) (125, P = 0.11; 175, P = 0.80; 275, P = 0.85; 575, P = 0.85; unpaired two-tailed t-test). Mean ± s.e.m. i, j, Inhibitory synaptic tuning in experienced cortex (i, n = 6 cells) and naive cortex (j, n = 6 cells; repeated measures one-way ANOVA, Bonferroni correction). k, Correlation of IPSCs with ISIs in experienced (left, n = 6 cells; Pearson’s r = 0.33, P = 0.11) and naive cortex (right, n = 6; Pearson’s r = 0.13, P = 0.56; two-tailed). l, Within cell comparison of PSCs in experienced cortex (125, P = 0.47; 175, P = 0.26; 275, P = 0.44; 575, P = 0.03; two-tailed paired t-test). *P < 0.05, **P < 0.01.
Extended Data Fig. 7 Variability in prototype-responsive neurons and single-cell temporal tuning curves during co-housing.
a, Example tracking of excitatory (top) and inhibitory (bottom) neurons. Coloured cell bodies denote prototype-responsive cells in that imaging session based on ∆F/F (%). b, c, Correlating prototype-evoked ∆F/F before (naive) and after retrieval onset (+24–96 h). Zeros denote non-responsive cells. Of the neurons that were prototype-responsive at baseline and successfully tracked, approximately 18.8% of excitatory neurons and 72.2% of inhibitory neurons remained responsive in the final imaging session. b, Excitatory (n = 104 neurons), Spearman’s r = −0.40, P < 0.0001 (two-tailed). c, Inhibitory (n = 118 neurons), Spearman’s r = 0.47, P < 0.0001 (two-tailed). d, Example excitatory neurons depicting single-cell dynamics during co-housing. Each set of three graphs is a single neuron’s tuning at baseline (naive), first retrieval, and 24 h after first retrieval (+24 h). Open circles denote neurons that were not prototype-responsive at a given time point, whereas ‘absent’ indicates a neuron that was not present in the imaging region. Colours represent ISIs used throughout the manuscript (prototype is in pink). e, As in d, but for inhibitory neurons.
Extended Data Fig. 8 Re-tuning of cortical neurons requires co-housing and reflects the statistics of pup call exemplars.
a, Excitatory temporal tuning before and after retrieval onset (raw data for Fig. 3e). Naive: n = 165 single-cell tuning curves, retrieving: n = 70. Mean ± s.e.m. b, As in a for interneurons. Naive: n = 122, retrieving: n = 82. c, d, To ensure that listening to pup calls while head-fixed under the two-photon microscope could not explain the broadening of excitatory tuning we observed in Fig. 3, we assessed temporal tuning to pup calls in pup-naive virgins on three consecutive imaging days without co-housing or retrieval testing. There were no systematic changes in cortical tuning in the absence of experience with pups (c, raw tuning; d, normalized tuning). Mean ± s.e.m. d, Session 1: n = 4 mice, n = 53 single-cell tuning curves; session 3: n = 5, n = 48 (one-way ANOVA, Bonferroni correction). e, Raw temporal tuning from virgins in Fig. 4b. Virgins were exposed to slow (ISI of 575 ms) morphs during co-housing (n = 6 mice). Tuning was assessed 24 h after retrieval onset; each coloured line represents an individual mouse’s tuning curve. f, Latency to retrieve on successful trials from Fig. 4c. CH (n = 35 trials) vs CH+575 (n = 29), P = 0.70 (unpaired two-tailed Mann–Whitney test). Median ± interquartile range. g, Latency to retrieve on successful trials from Fig. 4d. CH+575sham (n = 26 trials) vs CH+575opto-ACtx (n = 23), P = 0.19 (unpaired two-tailed Mann–Whitney test). Median ± interquartile range. h, i, Playback of single syllable calls during co-housing (‘CH+SS’) did not alter the behavioural-salience of single syllables. h, Retrieval rates in cold pup assay. CH (n = 6–18 mice), CH+SS (n = 4). Warm pup: CH (75.0%, n = 28 trials) vs CH+SS (85.7%, n = 14), P = 0.69. Single syllable: CH (n = 22.2%, n = 9) vs CH+SS (25.0%, n = 16), P > 0.99 (two-tailed Fisher’s exact test). Retrieval rate ± 95% binomial confidence intervals. i, Latency to retrieve on successful trials from h (CH, n = 23 trials; CH+SS, n = 16; P = 0.19; unpaired two-tailed Mann–Whitney test). Median ± interquartile range. *P < 0.05, **P < 0.01.
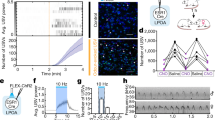
Extended Data Fig. 9 Optical inhibition of OT neurons perturbs the re-tuning of auditory cortical neurons.
a, Example in vitro current-clamp recording from pup-naive auditory cortex. The probability of evoking spikes in response to five extracellular stimulus pulses (ISI of 575 ms) was measured before and after an oxytocin (1 μM) wash. b, Left, evoked spike probability was significantly enhanced 15–30 min after the onset of oxytocin wash (n = 9 cells; P = 0.34 (stim 1), P = 0.01 (stim 2), P = 0.01 (stim 3), P = 0.001 (stim 4), P = 0.02 (stim 5)). Right, repetitive stimulation in the absence of oxytocin (ACSF, n = 7 cells) did not induce changes in spike probability (all stim, P > 0.99; one-way Friedman test with Dunn’s correction). Mean ± s.e.m. c, Example in vitro current-clamp recording from an oxytocin neuron containing halorhodopsin (eNpHR3.0). Photostimulation efficiently perturbed spiking for the duration of the light. d, Latency to retrieve on successful trials from Fig. 4e (CH+575sham (n = 32 trials) vs CH+575opto-OT (n = 20), P = 0.0003; unpaired two-tailed Mann–Whitney test). Median ± interquartile range. e, Temporal tuning curves from CH+575opto-OT virgins in Fig. 4f (n = 4 mice). Each coloured line represents the tuning curve for one virgin.
Extended Data Fig. 10 Intrinsic tuning in auditory cortex acts as a scaffold for experience-dependent plasticity during co-housing.
a, e, Synaptic tuning. b, c, f, g, Neuronal tuning. Colours denote ISIs used throughout the manuscript (red denotes fast, pink denotes prototypes, blue denotes slow). a, b, In pup-naive auditory cortex, excitatory neurons respond robustly to prototypical calls (a) as a result of sharply tuned excitatory drive and weak, untuned synaptic inhibition (b). Intrinsic tuning might result from hardwired cortical circuits or developmental experiences. c, Interneurons exhibit broad, unselective tuning characteristic of inhibitory populations that pool local activity. d, Oxytocin release during co-housing, possibly stimulated by virgin-pup interactions, may serve to transiently decrease intracortical inhibition8. This could enable the re-balancing of excitatory-inhibitory inputs: excitatory neurons broaden as excitatory drive increases across all ISIs. While inhibitory output tuning sharpens, net postsynaptic inhibitory drive is enhanced to (1) balance the increase in excitation across salient ISIs8 and (2) sharpen tuning to slow ISIs (e, I > E for slow ISIs). As a result of oxytocin receptor lateralization8, left auditory cortex may be particularly sensitive to exemplars frequently heard during co-housing. Whereas oxytocin may disinhibit the network, proper spike timing in relation to pup vocalizations is also required for the pairing of reliable pre- and post-synaptic activity27 (Fig. 4d). e–g, In experienced virgins, excitatory and inhibitory neurons are broadly tuned to behaviourally-salient ISIs, which reflect exemplar statistics, to enable generalization and reliable pup retrieval.
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
Schiavo, J.K., Valtcheva, S., Bair-Marshall, C.J. et al. Innate and plastic mechanisms for maternal behaviour in auditory cortex. Nature 587, 426–431 (2020). https://doi.org/10.1038/s41586-020-2807-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2807-6
This article is cited by
-
Social buffering in rats reduces fear by oxytocin triggering sustained changes in central amygdala neuronal activity
Nature Communications (2024)
-
The neural circuit that makes maternal mice respond to pups’ cries
Nature (2023)
-
Neural circuitry for maternal oxytocin release induced by infant cries
Nature (2023)
-
Neural circuit control of innate behaviors
Science China Life Sciences (2022)
-
Oxytocin neurons enable social transmission of maternal behaviour
Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.