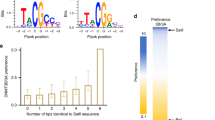
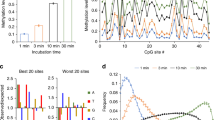
Abstract
CpG methylation by de novo DNA methyltransferases (DNMTs) 3A and 3B is essential for mammalian development and differentiation and is frequently dysregulated in cancer1. These two DNMTs preferentially bind to nucleosomes, yet cannot methylate the DNA wrapped around the nucleosome core2, and they favour the methylation of linker DNA at positioned nucleosomes3,4. Here we present the cryo-electron microscopy structure of a ternary complex of catalytically competent DNMT3A2, the catalytically inactive accessory subunit DNMT3B3 and a nucleosome core particle flanked by linker DNA. The catalytic-like domain of the accessory DNMT3B3 binds to the acidic patch of the nucleosome core, which orients the binding of DNMT3A2 to the linker DNA. The steric constraints of this arrangement suggest that nucleosomal DNA must be moved relative to the nucleosome core for de novo methylation to occur.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Density maps and structure coordinates have been deposited in the Electron Microscopy Data Bank with accession codes EMD-20281 and EMD-21689 and the Protein Data Bank with accession code 6PA7. The DNA methylation and MNase data have been deposited in the GEO database with accession code GSE152640. No restrictions are placed on data availability. Source data are provided with this paper.
References
Jones, P. A. & Baylin, S. B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 3, 415–428 (2002).
Jones, P. A. & Liang, G. Rethinking how DNA methylation patterns are maintained. Nat. Rev. Genet. 10, 805–811 (2009).
Takeshima, H., Suetake, I. & Tajima, S. Mouse Dnmt3a preferentially methylates linker DNA and is inhibited by histone H1. J. Mol. Biol. 383, 810–821 (2008).
Kelly, T. K. et al. Genome-wide mapping of nucleosome positioning and DNA methylation within individual DNA molecules. Genome Res. 22, 2497–2506 (2012).
Bourc’his, D., Xu, G. L., Lin, C. S., Bollman, B. & Bestor, T. H. Dnmt3L and the establishment of maternal genomic imprints. Science 294, 2536–2539 (2001).
Duymich, C. E., Charlet, J., Yang, X., Jones, P. A. & Liang, G. DNMT3B isoforms without catalytic activity stimulate gene body methylation as accessory proteins in somatic cells. Nat. Commun. 7, 11453 (2016).
Jia, D., Jurkowska, R. Z., Zhang, X., Jeltsch, A. & Cheng, X. Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature 449, 248–251 (2007).
Ooi, S. K. et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 448, 714–717 (2007).
Guo, X. et al. Structural insight into autoinhibition and histone H3-induced activation of DNMT3A. Nature 517, 640–644 (2015).
Zhang, Z. M. et al. Structural basis for DNMT3A-mediated de novo DNA methylation. Nature 554, 387–391 (2018).
Bowman, R. L., Busque, L. & Levine, R. L. Clonal hematopoiesis and evolution to hematopoietic malignancies. Cell Stem Cell 22, 157–170 (2018).
Heyn, P. et al. Gain-of-function DNMT3A mutations cause microcephalic dwarfism and hypermethylation of Polycomb-regulated regions. Nat. Genet. 51, 96–105 (2019).
Sendžikaitė, G., Hanna, C. W., Stewart-Morgan, K. R., Ivanova, E. & Kelsey, G. A DNMT3A PWWP mutation leads to methylation of bivalent chromatin and growth retardation in mice. Nat. Commun. 10, 1884 (2019).
Tatton-Brown, K. et al. Mutations in the DNA methyltransferase gene DNMT3A cause an overgrowth syndrome with intellectual disability. Nat. Genet. 46, 385–388 (2014).
Lowary, P. T. & Widom, J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 276, 19–42 (1998).
Kareta, M. S., Botello, Z. M., Ennis, J. J., Chou, C. & Chédin, F. Reconstitution and mechanism of the stimulation of de novo methylation by human DNMT3L. J. Biol. Chem. 281, 25893–25902 (2006).
Rhee, I. et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 416, 552–556 (2002).
Egger, G. et al. Identification of DNMT1 (DNA methyltransferase 1) hypomorphs in somatic knockouts suggests an essential role for DNMT1 in cell survival. Proc. Natl Acad. Sci. USA 103, 14080–14085 (2006).
Yang, X. et al. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell 26, 577–590 (2014).
Rondelet, G., Dal Maso, T., Willems, L. & Wouters, J. Structural basis for recognition of histone H3K36me3 nucleosome by human de novo DNA methyltransferases 3A and 3B. J. Struct. Biol. 194, 357–367 (2016).
Dukatz, M. et al. H3K36me2/3 binding and DNA binding of the DNA methyltransferase DNMT3A PWWP domain both contribute to its chromatin interaction. J. Mol. Biol. 431, 5063–5074 (2019).
Weinberg, D. N. et al. The histone mark H3K36me2 recruits DNMT3A and shapes the intergenic DNA methylation landscape. Nature 573, 281–286 (2019).
Qiu, C., Sawada, K., Zhang, X. & Cheng, X. The PWWP domain of mammalian DNA methyltransferase Dnmt3b defines a new family of DNA-binding folds. Nat. Struct. Biol. 9, 217–224 (2002).
Chen, T., Tsujimoto, N. & Li, E. The PWWP domain of Dnmt3a and Dnmt3b is required for directing DNA methylation to the major satellite repeats at pericentric heterochromatin. Mol. Cell. Biol. 24, 9048–9058 (2004).
Ge, Y. Z. et al. Chromatin targeting of de novo DNA methyltransferases by the PWWP domain. J. Biol. Chem. 279, 25447–25454 (2004).
Zhang, Y. et al. Chromatin methylation activity of Dnmt3a and Dnmt3a/3L is guided by interaction of the ADD domain with the histone H3 tail. Nucleic Acids Res. 38, 4246–4253 (2010).
Klimasauskas, S., Kumar, S., Roberts, R. J. & Cheng, X. HhaI methyltransferase flips its target base out of the DNA helix. Cell 76, 357–369 (1994).
Song, J., Rechkoblit, O., Bestor, T. H. & Patel, D. J. Structure of DNMT1-DNA complex reveals a role for autoinhibition in maintenance DNA methylation. Science 331, 1036–1040 (2011).
Henikoff, J. G., Belsky, J. A., Krassovsky, K., MacAlpine, D. M. & Henikoff, S. Epigenome characterization at single base-pair resolution. Proc. Natl Acad. Sci. USA 108, 18318–18323 (2011).
Chodavarapu, R. K. et al. Relationship between nucleosome positioning and DNA methylation. Nature 466, 388–392 (2010).
Barisic, D., Stadler, M. B., Iurlaro, M. & Schübeler, D. Mammalian ISWI and SWI/SNF selectively mediate binding of distinct transcription factors. Nature 569, 136–140 (2019).
Lyons, D. B. & Zilberman, D. DDM1 and Lsh remodelers allow methylation of DNA wrapped in nucleosomes. eLife 6, e30674 (2017).
Goll, M. G. & Bestor, T. H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 74, 481–514 (2005).
Yu, W. et al. Genome-wide DNA methylation patterns in LSH mutant reveals de-repression of repeat elements and redundant epigenetic silencing pathways. Genome Res. 24, 1613–1623 (2014).
Simon, M. D. & Shokat, K. M. A method to site-specifically incorporate methyl-lysine analogues into recombinant proteins. Methods Enzymol. 512, 57–69 (2012).
Simon, M. D. et al. The site-specific installation of methyl-lysine analogs into recombinant histones. Cell 128, 1003–1012 (2007).
Bouazoune, K., Miranda, T. B., Jones, P. A. & Kingston, R. E. Analysis of individual remodeled nucleosomes reveals decreased histone-DNA contacts created by hSWI/SNF. Nucleic Acids Res. 37, 5279–5294 (2009).
Dyer, P. N. et al. Reconstitution of nucleosome core particles from recombinant histones and DNA. Methods Enzymol. 375, 23–44 (2004).
Luger, K., Rechsteiner, T. J. & Richmond, T. J. Preparation of nucleosome core particle from recombinant histones. Methods Enzymol. 304, 3–19 (1999).
Ma, H. et al. A D53 repression motif induces oligomerization of TOPLESS corepressors and promotes assembly of a corepressor-nucleosome complex. Sci. Adv. 3, e1601217 (2017).
Jeong, S. et al. Selective anchoring of DNA methyltransferases 3A and 3B to nucleosomes containing methylated DNA. Mol. Cell. Biol. 29, 5366–5376 (2009).
Sharma, S., De Carvalho, D. D., Jeong, S., Jones, P. A. & Liang, G. Nucleosomes containing methylated DNA stabilize DNA methyltransferases 3A/3B and ensure faithful epigenetic inheritance. PLoS Genet. 7, e1001286 (2011).
Zhou, W., Triche, T. J. Jr, Laird, P. W. & Shen, H. SeSAMe: reducing artifactual detection of DNA methylation by Infinium BeadChips in genomic deletions. Nucleic Acids Res. 46, e123 (2018).
Kastner, B. et al. GraFix: sample preparation for single-particle electron cryomicroscopy. Nat. Methods 5, 53–55 (2008).
Stark, H. GraFix: stabilization of fragile macromolecular complexes for single particle cryo-EM. Methods Enzymol. 481, 109–126 (2010).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protocols 10, 845–858 (2015).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Acknowledgements
Cryo-EM data was collected at the David Van Andel Advanced Cryo-Electron Microscopy Suite at the Van Andel Institute (VAI). We thank X. Meng for cryo-EM data collection and EM technical support, the HPC team at VAI for computational support, D. Nadziejka for language editing of the manuscript, the VAI genomics core for performing the Illumina Infinium methylation EPIC array assay and J.-H. Min (Washington State University) and S. Baylin (John Hopkins University) for the indicated plasmids. This work was supported by the VAI (H.E.X., K.M., P.A.J.) and US National Cancer Institute (NCI; grants R35CA209859 to G.L., K.M. and P.A.J. and R50CA243878 to M.L.).
Author information
Authors and Affiliations
Contributions
H.E.X., K.M. and P.A.J. designed research. T.-H.X. expressed and purified proteins, prepared and screened samples and collected and processed cryo-EM data. T.-H.X. and M.L. performed biochemistry experiments. T.-H.X. and X.E.Z. built the atomic models and performed the refinements. T.-H.X., M.L., X.E.Z., G.L., G.Z., H.E.X., K.M. and P.A.J. analysed data. H.E.X., K.M. and P.A.J. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Protein expression and nucleosome reconstitution.
a, Histone octamer expression and purification. Histones H3 and H4 and His6-Sumo-tagged H2A and H3 were coexpressed from a polycistronic expression vector. The octamer was purified by native Ni-affinity chromatography, and then cleavage of the His6-Sumo tags with ULP1 Sumo protease was followed by size exclusion chromatography (SEC). The SDS–PAGE gel shows analysis of histone octamer purification steps. b, SEC profile of histone octamer with indicated size standards. c, Native gel analysis of NCP reconstitution. NCPs reconstituted by double dialysis were separated by native gel electrophoresis (lane 1) together with a size standard (lane 2) and free nucleosomal DNA (lane 3). The gel was stained for both DNA (left) and protein (right). d, SEC profile of cleaved (red) and non-cleaved (blue) DNMT3 complex. e, SDS–PAGE analysis of GFP-tagged DNMT and untagged DNMT purification steps. The GFP tag allowed easy DNMT3B3 visualization in DNMT–NCP complexes. For gel source data, see Supplementary Fig. 3.
Extended Data Fig. 2 DNMT3A2, DNMT3B3 and NCP form a stable complex.
a, DNMT3 and NCP stably interact in an AlphaScreen luminescence proximity assay. Top panel, cartoon of AlphaScreen assay; bottom panel, AlphaScreen interaction data. The nucleosomal DNA of the NCP is biotinylated for immobilization on AlphaScreen streptavidin donor beads, and DNMT3B3 is His8-GFP-tagged for immobilization of Ni-chelating AlphaScreen acceptor beads. Donor beads contained a photosensitizer that, upon activation at 680 nm, converts ambient oxygen to singlet oxygen. If donor and acceptor beads are brought into close proximity by DNMT–NCP interaction, energy is transferred from singlet oxygen to thioxene derivatives in the acceptor beads, resulting in light emission at 520–620 nm. Data are mean ± s.e.m., n = 10. b, Increasing concentrations of GFP-tagged DNMT3A2–DNMT3B3 complex supershift the NCP band. M, DNA size marker. Panels 1–4 show native gel with indicated binding reactions in (1) the ethidium bromide channel to visualize the DNA of the NCP and (2) the fluorescence channel to visualize the GFP-tagged DNMT3A2–DNMT3B3 complex; (3) a merged image of the two channels (yellow bands indicates presence of both DNMT complex and NCP; and (4) gel stained with Coomassie blue. For gel source data, see Supplementary Fig. 4.
Extended Data Fig. 3 DNMT3A–DNMT3B3 methylation activity and nucleosome interaction.
a, Free DNA methylation activity of acidic-patch-interacting and control mutant DNMT3B3 proteins. Histone-free (naked) 301-bp nucleosomal DNA was incubated with the indicated DNMT3A2–DNMT3B3 complexes containing wild-type or mutant DNMT3B3. Shown is the percentage methylation. Data are mean with individual values, n = 2. The total number of CpGs in the DNA is 24, of which a subset was preferentially methylated. b, Binding strength and structure resolution of DNMT3A2–DNMT3B3–NCP complexes with varying linker DNA lengths. Left, competition binding curves. AlphaScreen interaction between biotin-tagged NCPs and His6-tagged DNMT3A2–DNMT3B3 in the presence of increasing concentrations of untagged DNMT3A2–DNMT3B3. Data were generated from 3 independent experiments (n = 3) and normalized in each group with highest data point as 100%. Right, IC50 calculated in each experiment and represented in bar graphs. Data are mean ± s.e.m., n = 3, P values determined by non-parametric test with Dunn’s multiple comparisons test. ns, not significant. The table below compares binding strengths (IC50) and cryo-EM resolution of DNMT3A2–DNMT3B3 complexes with NCPs containing different length DNAs. The 200-kV data were collected on a Talos Arctica microscope with a Falcon 3 detector and the 300-kV data on a Titan Krios microscope with a K2 detector.
Extended Data Fig. 4 Overall structure of DNMT bound to the nucleosome.
a, Left, local resolution map of DNMT–nucleosome core complex (global resolution of 2.94 Å); middle, cutaway view; right, corrected Fourier shell correlation (FSC) curves of the 3D reconstruction. b, Left, overview of DNMT–nucleosome complex with ADD domains; middle, cartoon representation. Right, overlay of distal ADD EM density with aligned ADD in the auto-inhibitory conformation from PDB 4U7P. The inhibitory ADD loop, residues 526–533, is highlighted in blue. c, Overall map fitting of the nucleosome, DNMT ADD-CD/CLD dimer and DNMT CD/CLD dimer with local resolution colour code. The resolutions were determined using the 0.143 FSC criterion. d, Density map of the CD–DNA interaction region generated in PyMOL and contoured at 8σ (left) and 5.5σ (right). The dashed line indicates the position of the TRD loop, which is only visible at the lower σ level and we have omitted in the final model. e, Density maps of the two SAH ligands generated in PyMOL and contoured at 8σ.
Extended Data Fig. 5 Cryo-EM 3D reconstruction and refinement.
a, Representative micrograph, with one particle highlighted in a green circle of 250 Å diameter. b, Representative 2D class averages. c, Workflow for cryo-electron microscopy data processing by cryoSPARC and RELION3.0. Boxed 3D classes were selected for further processing. The final global nominal resolution for the DNMT–NCP complex was 2.94 Å.
Extended Data Fig. 6 Focused refinement of DNMT and NCP.
About 559,000 good particles were selected from 3D reconstructions for further focused refinement. The final resolution for the DNMT3A2–DNMT3B3 complex including the ADD domains was 3.40 Å, for the DNMT3A2 CD–DNMT3B3 CLD complex was 3.24 Å and for the NCP was 2.78 Å.
Extended Data Fig. 7 Conformation and density asymmetry of linker DNA (CD-bound versus free) and CLD (NCP-bound versus free).
a, b, Structure models overlaid with density maps (grey mesh) generated in PyMOL and contoured at 8σ (a) and 6σ (b). Note that at the 8σ contoured level, complete density is only visible for the CD-bound linker DNA and the histone-octamer-bound CLD. c, While the CD-bound linker DNA adopts a single conformation, the unbound linker DNA adopts four distinguishable conformations. Left, composite model; right, four different conformations shown as cryo-EM densities with their relative frequencies. d, Model of the DNMT3A2–DNMT3B3 complex with extended linker DNA and 3A2 ADD domain in its active conformation. The ADD has been modelled by structural alignment with PDB 4U7T. The structure model is fully compatible with the extended linker DNA. e, Model of the DNMT3A2–DNMT3B3 complex with extended linker DNA and 3A2 ADD domain in the autoinhibitory conformation. The ADD has been modelled by structural alignment with PDB 4U7P. This conformation is not compatible with the extended linker DNA.
Extended Data Fig. 8 MNase footprinting assay.
a, b, NCP301 alone (a) or in complex with DNMT3A2–DNMT3B3 (b) was subjected to MNase footprinting followed by DNA isolation and sequencing. Sequence reads shown by IGV viewer were aligned to the 301-bp reference to reveal the position of DNMT3A2–DNMT3B3 on individual nucleosomal DNA molecules (green, A; red, T; blue, C; brown, G). Magenta oval, position of the histone octamer (Widom 601 147-bp nucleosomal sequence); cyan bars, flexible linker region. Dashed lines demarcate the 10-bp linker regions of NCP167, and red dashed boxes represent the main DNMT protection region (protection of the DNMT–NCP complex relative to the DNMT-free NCP). The percentages of reads covering the DNMT protection regions are shown in red.
Extended Data Fig. 9 Histone H3 K36cMe3 did not improve the stability of ADD domains.
a, The H3K36cMe3 methyl-lysine analogue (MLA) peptide and H2A–H2B–H3K36cMe3 MLA–H4 histone octamer, but not the wild-type (WT) octamer, are recognized by anti-H3K36Me3 antibody. Four titrations (H3K36me3 peptide) or three titrations (H3Kc36me3 octamer) with different amounts of proteins were performed. b, AlphaScreen interaction assay between His-tagged DNMT3A2–DNMT3B3 complexes and biotinylated NCPs. The histone H3 K36cMe3 methyl analogue does not increase DNMT3A2–DNMT3B–NCP binding in the reconstituted system. Data are mean ± s.e.m., n = 3. c, Cryo-EM structure of the DNMT3A2–DNMT3B–H3K36cMe3 MLA NCP. The structure shows no additional density relative to that of the WT NCP complex. The ADD density is still poor and no reliable PWWP density is observed. d, Structural alignment of the DNMT–NCP WT (grey) and DNMT–NCP K36cMe3 (yellow) density maps shows their similar overall architecture. For gel source data, see Supplementary Fig. 5.
Supplementary information
Supplementary Information
This file contains: Supplementary Fig. 1: Sequence alignment; Supplementary Figs S2-S5: uncropped gels and size marker indications related to Fig. 4b and Extended Data Figs. 1a, 1c, 1e, 2b, and 9a and Supplementary Table 1: List of primers.
Video 1
Cryo-EM density map for human DNMT3A (CD) and DNMT3B3 (CLD) with NCP. Cryo-EM map and molecular model fitted in transparent density. The same color code is used as in Fig. 1c.
Rights and permissions
About this article
Cite this article
Xu, TH., Liu, M., Zhou, X.E. et al. Structure of nucleosome-bound DNA methyltransferases DNMT3A and DNMT3B. Nature 586, 151–155 (2020). https://doi.org/10.1038/s41586-020-2747-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2747-1
This article is cited by
-
DNMT3L inhibits hepatocellular carcinoma progression through DNA methylation of CDO1: insights from big data to basic research
Journal of Translational Medicine (2024)
-
Structure-guided functional suppression of AML-associated DNMT3A hotspot mutations
Nature Communications (2024)
-
Partial erosion on under-methylated regions and chromatin reprogramming contribute to oncogene activation in IDH mutant gliomas
Epigenetics & Chromatin (2023)
-
Structural and mechanistic insights into the DNA glycosylase AAG-mediated base excision in nucleosome
Cell Discovery (2023)
-
Tracking chromatin state changes using nanoscale photo-proximity labelling
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.