Abstract
Persistent neural activity in cortical, hippocampal, and motor networks has been described as mediating working memory for transiently encountered stimuli1,2. Internal emotional states, such as fear, also persist following exposure to an inciting stimulus3, but it is unclear whether slow neural dynamics are involved in this process. Neurons in the dorsomedial and central subdivisions of the ventromedial hypothalamus (VMHdm/c) that express the nuclear receptor protein NR5A1 (also known as SF1) are necessary for defensive responses to predators in mice4,5,6,7. Optogenetic activation of these neurons, referred to here as VMHdmSF1 neurons, elicits defensive behaviours that outlast stimulation5,8, which suggests the induction of a persistent internal state of fear or anxiety. Here we show that in response to naturalistic threatening stimuli, VMHdmSF1 neurons in mice exhibit activity that lasts for many tens of seconds. This persistent activity was correlated with, and required for, persistent defensive behaviour in an open-field assay, and depended on neurotransmitter release from VMHdmSF1 neurons. Stimulation and calcium imaging in acute slices showed that there is local excitatory connectivity between VMHdmSF1 neurons. Microendoscopic calcium imaging of VMHdmSF1 neurons revealed that persistent activity at the population level reflects heterogeneous dynamics among individual cells. Unexpectedly, distinct but overlapping VMHdmSF1 subpopulations were persistently activated by different modalities of threatening stimulus. Computational modelling suggests that neither recurrent excitation nor slow-acting neuromodulators alone can account for persistent activity that maintains stimulus identity. Our results show that stimulus-specific slow neural dynamics in the hypothalamus, on a time scale orders of magnitude longer than that of working memory in the cortex9,10, contribute to a persistent emotional state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Code for data analysis and modelling portions of this paper has been made publicly available at https://github.com/DJALab/VMHdm_persistence.
References
Major, G. & Tank, D. Persistent neural activity: prevalence and mechanisms. Curr. Opin. Neurobiol. 14, 675–684 (2004).
Curtis, C. E. & Lee, D. Beyond working memory: the role of persistent activity in decision making. Trends Cogn. Sci. 14, 216–222 (2010).
Anderson, D. J. & Adolphs, R. A framework for studying emotions across species. Cell 157, 187–200 (2014).
Gross, C. T. & Canteras, N. S. The many paths to fear. Nat. Rev. Neurosci. 13, 651–658 (2012).
Kunwar, P. S. et al. Ventromedial hypothalamic neurons control a defensive emotion state. eLife 4, (2015).
Silva, B. A. et al. Independent hypothalamic circuits for social and predator fear. Nat. Neurosci. 16, 1731–1733 (2013).
Silva, B. A. et al. The ventromedial hypothalamus mediates predator fear memory. Eur. J. Neurosci. 43, 1431–1439 (2016).
Wang, L., Chen, I. Z. & Lin, D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron 85, 1344–1358 (2015).
Guo, Z. V. et al. Maintenance of persistent activity in a frontal thalamocortical loop. Nature 545, 181–186 (2017).
Inagaki, H. K., Fontolan, L., Romani, S. & Svoboda, K. Discrete attractor dynamics underlies persistent activity in the frontal cortex. Nature 566, 212–217 (2019).
Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014).
Papes, F., Logan, D. W. & Stowers, L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell 141, 692–703 (2010).
Yilmaz, M. & Meister, M. Rapid innate defensive responses of mice to looming visual stimuli. Curr. Biol. 23, 2011–2015 (2013).
Simon, P., Dupuis, R. & Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 61, 59–64 (1994).
Berndt, A. et al. Structural foundations of optogenetics: determinants of channelrhodopsin ion selectivity. Proc. Natl Acad. Sci. USA 113, 822–829 (2016).
Han, S., Soleiman, M. T., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374 (2015).
Dana, H. et al. Sensitive red protein calcium indicators for imaging neural activity. eLife 5, e12727 (2016).
Ziv, Y. et al. Long-term dynamics of CA1 hippocampal place codes. Nat. Neurosci. 16, 264–266 (2013).
Remedios, R. et al. Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 550, 388–392 (2017).
Ben-Shaul, Y., Katz, L. C., Mooney, R. & Dulac, C. In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc. Natl Acad. Sci. USA 107, 5172–5177 (2010).
Mongeau, R., Miller, G. A., Chiang, E. & Anderson, D. J. Neural correlates of competing fear behaviors evoked by an innately aversive stimulus. J. Neurosci. 23, 3855–3868 (2003).
Arch, V. S. & Narins, P. M. “Silent” signals: selective forces acting on ultrasonic communication systems in terrestrial vertebrates. Anim. Behav. 76, 1423–1428 (2008).
McCormick, D. A. Brain calculus: neural integration and persistent activity. Nat. Neurosci. 4, 113–114 (2001).
Goldman, M., Compte, A. & Wang, X.-J. in Encyclopedia of Neuroscience (ed. Squire, L. R.) 1–26 (Elsevier, 2007).
Wang, X.-J. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 24, 455–463 (2001).
Jan, Y. N., Jan, L. Y. & Kuffler, S. W. Further evidence for peptidergic transmission in sympathetic ganglia. Proc. Natl Acad. Sci. USA 77, 5008–5012 (1980).
Wang, X. J. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J. Neurosci. 19, 9587–9603 (1999).
Sompolinsky, H., Crisanti, A. & Sommers, H.-J. Chaos in random neural networks. Phys. Rev. Lett. 61, 259–262 (1988).
Toyoizumi, T. & Abbott, L. F. Beyond the edge of chaos: amplification and temporal integration by recurrent networks in the chaotic regime. Phys. Rev. E 84, 051908 (2011).
Viskaitis, P. et al. Modulation of SF1 neuron activity coordinately regulates both feeding behavior and associated emotional states. Cell Rep. 21, 3559–3572 (2017).
Jan, Y. N., Jan, L. Y. & Kuffler, S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc. Natl Acad. Sci. USA 76, 1501–1505 (1979).
Zelikowsky, M. et al. The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell 173, 1265–1279.e1219 (2018).
Kim, D.-W. et al. Multimodal analysis of cell types in a hypothalamic node controlling social behavior. Cell 179, 713–728.e17 (2019).
Bellot-Saez, A., Kékesi, O., Morley, J. W. & Buskila, Y. Astrocytic modulation of neuronal excitability through K+ spatial buffering. Neurosci. Biobehav. Rev. 77, 87–97 (2017).
Motta, S. C. et al. Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc. Natl Acad. Sci. USA 106, 4870–4875 (2009).
Miller, S. M., Marcotulli, D., Shen, A. & Zweifel, L. S. Divergent medial amygdala projections regulate approach-avoidance conflict behavior. Nat. Neurosci. 22, 565–575 (2019).
Dhillon, H. et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49, 191–203 (2006).
Xu, X. et al. Modular genetic control of sexually dimorphic behaviors. Cell 148, 596–607 (2012).
Yang, C. F. et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909 (2013).
Correa, S. M. et al. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep. 10, 62–74 (2015).
Ishii, K. K. et al. A labeled-line neural circuit for pheromone-mediated sexual behaviors in mice. Neuron 95, 123–137.e128 (2017).
Lerner, T. N. et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015).
Lu, J. et al. MIN1PIPE: a miniscope 1-photon-based calcium imaging signal extraction pipeline. Cell Rep. 23, 3673–3684 (2018).
Franklin, K. B. J. & Paxinos, G. The Mouse Brain in Stereotactic Coordinates 3rd edn (2008).
Yang, L., Lee, K., Villagracia, J. & Masmanidis, S. C. Open source silicon microprobes for high throughput neural recording. J. Neural Eng. 17, 016036 (2020).
Zhou, P. et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife 7, e28728 (2018).
Giovannucci, A. et al. CaImAn an open source tool for scalable calcium imaging data analysis. eLife 8, e38173 (2019).
Walz, W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int. 36, 291–300 (2000).
Acknowledgements
We thank R. Palmiter for providing TetTox–GFP in advance of publication; H. Inagaki, M. Meister, L. F. Abbott, U. Rutishauser, and members of the Anderson lab for helpful comments on the manuscript; R. Remedios for help with miniscope imaging; L. Liu for teaching silicon probe recording; A. Vinograd for preliminary imaging experiments; T. Davidson and K. Deisseroth for teaching fibre photometry; B. Yang for helping with the TetTox fibre photometry experiment; X. Da, J. S. Chang and C. Kim for technical assistance, X. Da and C. Chiu for laboratory management and G. Mancuso for administrative support. This work was supported by NIH Grant R01 MH112593. K99 MH117264 to A.K., a Helen Hay Whitney Foundation Postdoctoral Fellowship to L.L., and the EMBO ALTF 736-2018 to S.S. D.J.A. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
A.K., P.S.K., and D.J.A. conceptualized and designed the fibre photometry and microendoscopic imaging experiments. A.K., L.L., P.S.K., and D.J.A. conceptualized and designed the optogenetic loss-of-function experiments. D.A.W. and P.S.K. designed and built the imaging acquisition setup. P.S.K. performed imaging experiments. L.L. performed loss-of-function, rat exposure assay and imaging, tetanus toxin, and extracellular recording experiments. S.S. performed brain slice electrophysiology and Ca2+ imaging experiments and analysis. A.K. performed imaging data analysis and modeling. A.K., P.S.K., L.L., and D.J.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Jaideep Bains, Larry Zweifel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Additional properties of VMHdmSF1+ neurons’ responses to rat, rat urine, and looming disk stimuli.
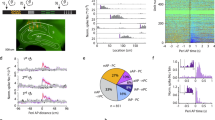
a, Peak ∆F/F activity in response to rat in home cage (anaesthetized, uncaged stimulus) and a head-fixed set-up (awake, caged stimulus). (home cage group n = 4 mice; head-fixed group n = 8 mice; mean ± s.e.m.). b, Decay time to 10% of peak (same mice as a; mean ± s.e.m.). c, Rise time constant of rat-evoked activity. (same mice as a; mean ± s.e.m.). d, Decay time constant of rat-evoked activity. (same mice as a; mean ± s.e.m.). e, Schematic illustrating urine presentation to head-fixed mouse for fibre photometry. f, Averaged ∆F/F activity traces of SF1+ neurons in response to rat urine or water (n = 6 mice, 2 trials per mouse; mean ± s.e.m.). g, Peak ∆F/F activity triggered by rat urine or water. (same mice as f; mean ± s.e.m.; P = 0.0001, paired t-test). h, Decay time constant for rat or rat urine response (n = 8 mice (rat), n = 6 mice (urine), 2 trials per mouse; mean ± s.e.m.; P = 0.025, t-test). i, Looming disk presentation to head-fixed mouse for fibre photometry. j, ∆F/F response to rat, toy rat, or looming disk stimuli presented for 10 s in the animal’s home cage (n = 4 mice, 1 trial per mouse; mean ± s.e.m.). k, Peak of ∆F/F response to rat, toy rat, and looming disk stimuli. (n same as in j; mean ± s.e.m.; rat vs toy, P = 0.04; rat vs looming disk, P = 0.0273, paired t-test).
Extended Data Fig. 2 No change in mouse behaviour due to potential lingering odor from rat.
a, Schematic plot showing experiment protocol: top, a live rat or toy rat (control) was brought to the open field arena in a wire mesh cage for 15 s; bottom, mouse was introduced to arena afterwards immediately. b, Fraction of time spent in edge zone (defined by red dashed line in a) for rat group and control group (n = 6 for each group; mean ± s.e.m.). c, Distance from mouse body centre to arena centre. (same mice as b; mean ± s.e.m.). d, Schematic plot showing optogenetic activation protocol. Mice expressing ChR2 in VMHdmSF1 neurons were introduced to the open field arena. After a five-minute habituation period, a ten-second light or mock stimulation was delivered to the mice. e, Fraction of time spent in the edge zone. Dashed lines mark time of rat presentation. (n = 4 mice for each group; mean ± s.e.m.; P = 0.0012, repeated measures ANOVA test).
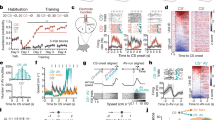
Extended Data Fig. 3 Fibre photometry and VMHdmSF1 neuron silencing in open field rat exposure assay.
a, Fraction of time spent in edge zone during three different time periods: before rat, after rat and after photostimulation offset, corresponding to –1 - 0, 0 - 1 and 3 - 4 min intervals in Fig. 1l. (same mice as Fig. 1l; mean ± s.e.m.; control, before vs after rat, P = 1.8e-05, paired t-test; after rat, control vs PS during + after, P = 5.2e-05, t-test; after rat, control vs PS after, P = 0.025, t-test; after PS off, control vs PS during + after, P = 0.024, t-test; after PS off, control vs PS after only, P = 0.046, t-test). b, Mean velocity was not altered by photostimulation of iC++ and control (Cre-dependent iC++ virus injected into wild type littermate) mice. Velocity was measured in mouse home cage and averaged during a three-minute period for light off and light on sessions. (n = 7 mice; iC++, light off vs light on, P = 0.7059; control, light off vs light on, p = 0.3537, paired t-test). c, ∆F/F activity traces (mean ± s.e.m.) of VMHdmSF1 neurons in response to rat presentation in open field arena. Shaded grey bar denotes the 15 s presentation of rat (n = 9 mice). d, Peak ∆F/F activity triggered by awake, caged rat in open field arena (n = 9) and head-fixed set up (n = 8) (mean ± s.e.m.; P = 0.4681, t-test). e, Decay constants of ∆F/F activity in open filed arena compared to head-fixed set up (same mice as d; mean ± s.e.m.; P = 0.3633, t-test). f, Comparison of traces (mean ± s.e.m.) for ∆F/F activity (blue) and the distance from mouse body centre to arena centre (orange), aligned to time of rat removal. (n = 9 mice; distance to centre is plotted as a 30-s moving average.) g, Decay time measured as the time elapsed to reach 50% of the peak for linearly fitted data. (n = 9; mean ± s.e.m.; P = 0.4476, paired t-test). h, Scatter plot of ∆F/F activity vs. distance from mouse to arena centre, fit by linear regression, for two example mice. Mouse 1, r = 0.958, P < 0.0001; mouse 2, r = 0.808, P < 0.0001. i, Pearson’s correlation coefficient between ∆F/F activity and the distance to centre, with the two mice plotted in (h) indicated by colored arrowheads (n = 9 mice; mean ± s.e.m.). j, Additional control for optogenetic loss-of-function experiment (see Fig. 1l), using Cre-dependent AAV-DIO-eYFP virus injected into SF1-Cre mice. Green horizontal bar denotes photostimulation period (n = 5, eYFP group; n = 6, iC++ group (SF1-Cre mice injected with AAV-DIO-iC++). P = 0.0433, repeated measures ANOVA test, mean ± s.e.m.).
Extended Data Fig. 4 Excitatory monosynaptic interconnectivity in VMHdmSF1 neurons, sensitive to glutamate receptor blockade.
a, Schematic illustration of the experimental design used to transduce the majority of VMHdmSF1 neurons with Cre-dependent GCaMP7s and a minority of VMHdmSF1 neurons with Cre-dependent ChrimsonR-tdTomato, for the study of functional connectivity in VMHdm. b, Schematic illustration of the experimental design used to identify functional connectivity among VMHdmSF1 neurons using whole-cell patch-clamp recordings guided by differential expression of GCaMP and ChrimsonR-tdTomato. c, Maximum projection confocal image of a VMHdmSF1 neuron recorded ex vivo, and filled with Neurobiotin conjugated to a far red fluorophore (AlexaFluor647; n = 7 neurons recorded and filled in 7 slices from 5 mice). d, Left – Average of voltage-clamp recordings at the reversal of inhibition (VHold = -70 mV) indicative of a post-synaptic response following photostimulation of ChrimsonR (blue line), sensitive to glutamate receptor blockage (black line; n = 7 cells from 5 mice, 6/7 cells connected; mean ± s.e.m.). Middle – Quantification of the optically evoked excitatory post-synaptic current in control, vs glutamate receptor blockade conditions (n = 7 cells per condition, P = 0.0041, two-tailed paired t-test, box plot elements for control condition; minimum = -31.10 pA, 25% percentile = -26.60 pA, median = -20.10 pA, 75% percentile = -11.80 pA, maximum = -0.1 pA, box plot elements for glutamate receptor blockade condition; minimum = -2.10 pA, 25% percentile = -0.90 pA, median = 0.20 pA, 75% percentile = 1.60 pA, maximum = 2.2 pA). Right – Frequency distribution of the optically evoked excitatory post-synaptic currents in a 15 ms window. e, Ex vivo single neuron whole-cell patch-clamp electrophysiology and Ca2+ imaging. Left column – Top, presentation of current-clamp recording during which a neuron from the field of view is clamped at -70 mV and depolarizing square pulses are delivered to induce action potential firing. Left column – Bottom, raster plot of Ca2+ imaging recordings identifying Ca2+ responsive cells following electrical stimulation of the patch-clamped neuron (highlighted by the magenta circle, neuron #90). Several other cells respond with an increase in their Ca2+ activity following electrical stimulation (highlighted by colored circles on the top right side of the activity colour plot, neurons #87, #85, #82, #80, #78 and #66. Right – expanded view of the electrophysiology and superimposed imaging traces from four stimulation trials. f, Example cross-correlation colour plot of the Ca2+ activity of the patch-clamped neuron (in this plot Cell #1), against the recorded Ca2+ activity of thirteen other VMHdmSF1 neurons. g, Quantification of follower cells per brain slice, identified as neurons with cross-correlation coefficient >0.6 compared vs. the Ca2+ trace of the electrically stimulated neuron (n = 5 brain slices from 5 mice, box plot elements; minimum = 2, 25% percentile = 2.5, median = 4, 75% percentile = 6.5, maximum = 8).
Extended Data Fig. 5 Summary of fibre/GRIN lens placements.
a, Map of the recording sites for fibre-photometry mice included in Fig. 1. b, Map of the microscope GRIN lens location for mice illustrated in Figs. 2–3. c, Map of the fibre tip locations in optogenetic silencing (iC++) mice illustrated in Fig. 1. d, Map of the recording sites for tetanus toxin light chain (TTX) experiment mice illustrated in Fig. 1. Anatomical images from44.
Extended Data Fig. 6 Confirmation of VMHdm/c population dynamics using in vivo electrophysiology.
a, Schematic illustrating silicon probe recording from VMHdm/c in head-fixed mouse. b, Histogram of the spontaneous firing rate of all recorded cells in VMHdm. Red dotted line indicates that 90% of cells have a spontaneous firing rate ≤ 13Hz. c, Percent of cells excited, inhibited, or not responsive to rat. A similar percentage of rat-responsive cells was detected by microendoscopic imaging of calcium activity (Fig. 3l). d, Mean population firing rate evoked by rat. All firing rates in this figure were estimated in one-second time bins. (n = 5 mice, mean ± s.e.m.). e, Rat evoked responses in six example cells. Left, colour map showing the normalized firing rate of individual cells on each of five repeated trials. White dotted lines mark the duration of rat presentation. Right, traces showing the average firing rate over the five trials (mean ± s.e.m.). f, Trial averaged, normalized firing rates of rat-responsive cells, sorted by time of response peak. g, Histogram of times to peak firing rate for rat responsive cells; compare to Fig. 2j (n = 370 cells from 5 mice). h, Histogram of times of decay to half of the peak firing rate for rat responsive cells, compare to Fig. 2m (n same as in g). i, Scatter plot comparing cell responses at 2 or 20 s after rat introduction (n same as in g).
Extended Data Fig. 7 Stability of the VMHdmSF1 population response across trials and days.
a, Responses of ten example VMHdmSF1 neurons across three days of imaging, from the n = 5 microendoscopic imaging mice. The five stimuli are presented for two trials (tr1, tr2) each day in pseudorandomized order, with ten minutes between stimulus presentations, on 3 consecutive days. Some cells show strong, consistent tuning across all trials/days (cells 1-4). Other neurons show consistent tuning, but have trial-to-trial variability in response sizes (cells 5-7). Others show adaptation of their responses across trials and days (cells 8-10). b, Population mean response to different stimuli on each trial across three days of imaging, showing a decrease in the population response across trials and days. (n = 5 mice, mean ± s.e.m.) c, Pearson’s correlation between stimulus-evoked population activity on day 2 vs day 3 of imaging (n = 5 mice, mean ± s.e.m.). While there is some trial-to-trial and day-to-day variability in cells’ responses, stimulus identity is maintained by the population across days: this is reflected by the higher Pearson’s correlation of a stimulus with itself than with other stimuli, and by the accuracy of decoders trained to predict stimulus identity from population activity (see Fig. 3m-n, Extended Data Fig. 8). d, Matrix of Pearson’s correlation between the mean population responses to all stimuli on day A and the responses on day B, for days 1 vs 2, days 2 vs 3, and days 1 vs 3 (mean across n = 5 mice). e, Pearson’s correlation between each cell’s time-averaged response to all five stimuli on day A vs that cell’s responses on day B, plotted against that cell’s response to its most strongly preferred stimulus. Cells with small max responses (lower y-axis values) can show variability in their activity from day to day (reflected in a lower Pearson’s correlation on the x-axis), while cells that show strong responses to one or more stimuli (higher y-axis values) tend to be more consistent in their stimulus tuning from day to day (higher PCC).
Extended Data Fig. 8 Additional Pearson’s correlations between stimulus pairs.
Pearson’s correlation between VMHdmSF1 population activity as a function of time, evoked by all possible pairs of stimuli (n = 5 imaged mice; mean ± s.e.m.).
Extended Data Fig. 9 Additional decoder analysis of VMHdmSF1 population activity.
a, Confusion matrix of the five-way Naïve Bayes decoder shown in Fig. 3m, showing predicted stimulus identity for each stimulus class. Matrix is normalized so rows sum to 100%. b, Accuracy of a time-dependent five-way Naïve Bayes decoder, as a function of time, for each tested stimulus. c, Accuracy of time-dependent binary Naïve Bayes decoders trained on all possible pairs of stimuli. The pair of stimuli being decoded for each plot is specified by the labels on the left and top. All plots show mean ± s.e.m. across five imaged mice. Dashed horizontal line indicates chance.
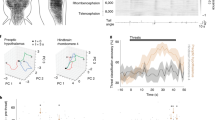
Extended Data Fig. 10 Locally connected model networks.
a, Probability of synapse formation between neuron pairs decreases moderately as a function of “distance” (neuron number) in the locally connected sRNN model. Segments of the model targeted by rat and USS model input are also shown (blue/purple lines.) b, Example synaptic weight matrix generated from probability matrix shown in a; for visibility every 10th model neuron is shown. c, Example of a more highly structured model network, in which largely separate populations of neurons respond to the rat vs USS model inputs. d, Pearson’s correlation (left graph) and stimulus-evoked autocorrelation (right graph) for a network model such as that in c, in which network structure results in no overlap between rat and USS representations, whereas the actual data (n = 5 mice; dashed black line with grey s.e.m. envelope; data reproduced from Fig. 4) shows partial overlap.
Supplementary information
Supplementary Table
Supplementary Table 1. Statistical reporting summary for all hypothesis testing in the paper. (pdf)
Rights and permissions
About this article
Cite this article
Kennedy, A., Kunwar, P.S., Li, Ly. et al. Stimulus-specific hypothalamic encoding of a persistent defensive state. Nature 586, 730–734 (2020). https://doi.org/10.1038/s41586-020-2728-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2728-4
This article is cited by
-
Independent inhibitory control mechanisms for aggressive motivation and action
Nature Neuroscience (2024)
-
The dynamic state of a prefrontal–hypothalamic–midbrain circuit commands behavioral transitions
Nature Neuroscience (2024)
-
Defensive responses: behaviour, the brain and the body
Nature Reviews Neuroscience (2023)
-
Neural circuitry for maternal oxytocin release induced by infant cries
Nature (2023)
-
Preserved neural dynamics across animals performing similar behaviour
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.