Abstract
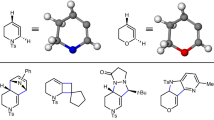
Strained cyclic organic molecules, such as arynes, cyclic alkynes and cyclic allenes, have intrigued chemists for more than a century with their unusual structures and high chemical reactivity1. The considerable ring strain (30–50 kilocalories per mole)2,3 that characterizes these transient intermediates imparts high reactivity in many reactions, including cycloadditions and nucleophilic trappings, often generating structurally complex products4. Although strategies to control absolute stereochemistry in these reactions have been reported using stoichiometric chiral reagents5,6, catalytic asymmetric variants to generate enantioenriched products have remained difficult to achieve. Here we report the interception of racemic cyclic allene intermediates in a catalytic asymmetric reaction and provide evidence for two distinct mechanisms that control absolute stereochemistry in such transformations: kinetic differentiation of allene enantiomers and desymmetrization of intermediate π-allylnickel complexes. Computational studies implicate a catalytic mechanism involving initial kinetic differentiation of the cyclic allene enantiomers through stereoselective olefin insertion, loss of the resultant stereochemical information, and subsequent introduction of absolute stereochemistry through desymmetrization of an intermediate π-allylnickel complex. These results reveal reactivity that is available to cyclic allenes beyond the traditional cycloadditions and nucleophilic trappings previously reported, thus expanding the types of product accessible from this class of intermediates. Additionally, our computational studies suggest two potential strategies for stereocontrol in reactions of cyclic allenes. Combined, these results lay the foundation for the development of catalytic asymmetric reactions involving these classically avoided strained intermediates.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre under CCDC 1987661. The authors declare that all other data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Wenk, H. H., Winkler, M. & Sander, W. One century of aryne chemistry. Angew. Chem. Int. Ed. 42, 502–528 (2003).
Liebman, J. F. & Greenberg, A. A survey of strained organic molecules. Chem. Rev. 76, 311–365 (1976).
Angus, R. O., Jr, Schmidt, M. W. & Johnson, R. P. Small-ring cyclic cumulenes: theoretical studies of the structure and barrier to inversion of cyclic allenes. J. Am. Chem. Soc. 107, 532–537 (1985).
Pellissier, H. & Santelli, M. The use of arynes in organic synthesis. Tetrahedron 59, 701–730 (2003).
Dockendorff, C., Sahli, S., Olsen, M., Milhau, L. & Lautens, M. Synthesis of dihydronaphthalenes via aryne-Diels–Alder reactions: scope and diastereoselectivity. J. Am. Chem. Soc. 127, 15028–15029 (2005).
Picazo, E. et al. Arynes and cyclic alkynes as synthetic building blocks for stereodefined quaternary centers. J. Am. Chem. Soc. 140, 7605–7610 (2018).
Stoermer, R. & Kahlert, B. Ueber das 1-Brom-cumaron. Ber. Dtsch. Chem. Ges. 35, 1633–1640 (1902).
Roberts, J. D., Simmons, H. E., Carlsmith, L. A. & Vaughan, C. W. Rearrangement in the reaction of chlorobenzene-1-C14 with potassium amide. J. Am. Chem. Soc. 75, 3290–3291 (1953).
Wittig, G. Phenyl-lithium, der Schlüssel zu einer neuen Chemie metallorganischer Verbindungen. Naturwissenschaften 30, 696–703 (1942).
Wittig, G. & Pohmer, L. Intermediäre Bildung von Dehydrobenzol (Cyclohexa-dienin). Angew. Chem. 67, 348 (1955).
Scardiglia, F. & Roberts, J. D. Evidence for cyclohexyne as an intermediate in the coupling of phenyllithium with 1-chlorocyclohexene. Tetrahedron 1, 343–344 (1957).
Wittig, G. & Fritze, P. On the intermediate occurrence of 1,2-cyclohexadiene. Angew. Chem. Int. Edn Engl. 5, 846 (1966).
Berry, R. S., Clardy, J. & Schafer, M. E. Benzyne. J. Am. Chem. Soc. 86, 2738–2739 (1964).
Diau, E. W.-G., Casanova, J., Roberts, J. D. & Zewail, A. H. Femtosecond observation of benzyne intermediates in a molecular beam: Bergman rearrangement in the isolated molecule. Proc. Nat. Acad. Sci. 97, 1376–1379 (2000).
Dubrovskiy, A. V., Markina, N. A. & Larock, R. C. Use of benzynes for the synthesis of heterocycles. Org. Biomol. Chem. 11, 191–218 (2013).
Chen, S. et al. Preparation of substituted 2,2-bipyrimidinyl compounds and analogs thereof, and methods using the same. International patent W02019222238 A2 (2019).
Mauger, C. C. & Mignani, G. A. An efficient and safe procedure for the large-scale Pd-catalyzed hydrazonation of aromatic chlorides using Buchwald technology. Org. Process Res. Dev. 8, 1065–1071 (2004).
Takikawa, H., Nishii, A., Sakai, T. & Suzuki, K. Aryne-based strategy in the total synthesis of naturally occurring polycyclic compounds. Chem. Soc. Rev. 47, 8030–8056 (2018).
Tadross, P. M. & Stoltz, B. M. A comprehensive history of arynes in natural product total synthesis. Chem. Rev. 112, 3550–3577 (2012).
Gampe, C. M. & Carreira, E. M. Arynes and cyclohexyne in natural product synthesis. Angew. Chem. Int. Ed. 51, 3766–3778 (2012).
Schleth, F., Vettiger, T., Rommel, M. & Tobler, H. Process for the preparation of pyrazole carboxylic acid amides. International patent WO2011131544 A1 (2011).
Lin, J. B., Shah, T. J., Goetz, A. E., Garg, N. K. & Houk, K. N. Conjugated trimeric scaffolds accessible from indolyne cyclotrimerizations: synthesis, structures, and electronic properties. J. Am. Chem. Soc. 139, 10447–10455 (2017).
Caeiro, J., Peña, D., Cobas, A., Pérez, D. & Guitián, E. Asymmetric catalysis in the [2+2+2] cycloaddition of arynes and alkynes: enantioselective synthesis of a pentahelicene. Adv. Synth. Catal. 348, 2466–2474 (2006).
Yubuta, A. et al. Enantioselective synthesis of triple helicenes by cross-cyclotrimerization of a helicenyl aryne and alkynes via dynamic kinetic resolution. J. Am. Chem. Soc. 142, 10025–10033 (2020).
Li, L., Li, Y., Fu, N., Zhang, L. & Luo, S. Catalytic asymmetric electrochemical α-arylation of cyclic β-ketocarbonyls with anodic benzyne intermediates. Angew. Chem. Int. Ed. 59, 14347–14351 (2020).
Quintana, I., Peña, D., Pérez, D. & Guitián, E. Generation and reactivity of 1,2-cyclohexadiene under mild reaction conditions. Eur. J. Org. Chem. 2009, 5519–5524 (2009).
Barber, J. S. et al. Diels–Alder cycloadditions of strained azacyclic allenes. Nat. Chem. 10, 953–960 (2018).
Lofstrand, V. A. & West, F. G. Efficient trapping of 1,2-cyclohexadienes with 1,3-dipoles. Chemistry 22, 10763–10767 (2016).
Barber, J. S. et al. Nitrone cycloadditions of 1,2-cyclohexadiene. J. Am. Chem. Soc. 138, 2512–2515 (2016).
Yamano, M. M. et al. Cycloadditions of oxacyclic allenes and a catalytic asymmetric entryway to enantioenriched cyclic allenes. Angew. Chem. Int. Ed. 58, 5653–5657 (2019).
Lofstrand, V. A., McIntosh, K. C., Almehmadi, Y. A. & West, F. G. Strain-activated Diels–Alder trapping of 1,2-cyclohexadienes: intramolecular capture by pendent furans. Org. Lett. 21, 6231–6234 (2019).
Pellissier, H. Dynamic kinetic resolution. Tetrahedron 59, 8291–8327 (2003).
Balci, M. & Jones, W. M. Chirality as a probe for the structure of 1,2-cycloheptadiene and 1,2-cyclohexadiene. J. Am. Chem. Soc. 102, 7607–7608 (1980).
Dhokale, R. A. & Mhaske, S. B. Transition-metal-catalyzed reactions involving arynes. Synthesis 50, 1–16 (2018).
Chattopadhyay, B. & Gevorgyan, V. Transition-metal-catalyzed denitrogenative transannulation: converting triazoles into other heterocyclic systems. Angew. Chem. Int. Ed. 51, 862–872 (2012).
Thorat, V. H., Upadhyay, N. S., Murakami, M. & Cheng, C.-H. Nickel-catalyzed denitrogenative annulation of 1,2,3-benzotriazin-4-(3H)-ones with benzynes for construction of phenanthridinone scaffolds. Adv. Synth. Catal. 360, 284–289 (2018).
Wang, N., Zheng, S.-C., Zhang, L.-L., Guo, Z. & Liu, X.-Y. Nickel(0)-catalyzed denitrogenative transannulation of benzotriazinones with alkynes: mechanistic insights of chemical reactivity and regio- and enantioselectivity from density functional theory and experiment. ACS Catal. 6, 3496–3505 (2016).
Yamauchi, M., Morimoto, M., Miura, T. & Murakami, M. Enantioselective synthesis of 3,4-dihydroisoquinolin-1(2H)-ones by nickel-catalyzed denitrogenative annulation of 1,2,3-benzotriazin-4(3H)-ones with allenes. J. Am. Chem. Soc. 132, 54–55 (2010).
Jin, Z. & Yao, G. Amaryllidaceae and sceletium alkaloids. Nat. Prod. Rep. 36, 1462–1488 (2019).
Miura, T., Yamauchi, M., Kosaka, A. & Murakami, M. Nickel-catalyzed regio- and enantioselective annulation reactions of 1,2,3,4-benzothiatriazine-1,1(2H)-dioxides with allenes. Angew. Chem. Int. Ed. 49, 4955–4957 (2010).
Feng, M., Tang, B., Wang, N., Xiu, H.-X. & Jiang, X. Ligand controlled regiodivergent C1 insertion on arynes for construction of phenanthridinone and acridone alkaloids. Angew. Chem. Int. Ed. 54, 14960–14964 (2015).
Bickelhaupt, F. M. & Houk, K. N. Analyzing reaction rates with the distortion/interaction activation strain model. Angew. Chem. Int. Ed. 56, 10070–10086 (2017).
Acknowledgements
We are grateful to the NIH-NIGMS (R01 GM123299 and R01 GM132432 for N.K.G., and T32 GM067555 for A.V.K.), the NSF (CHE-1764328 for K.N.H., DGE-1144087 for M.M.Y. and B.J.S., and DGE-1650604 for A.V.K.), the Trueblood family (for N.K.G.), and the Swiss National Science Foundation for an Early Mobility Postdoctoral Fellowship (M.G.). These studies were supported by shared instrumentation grants from the NSF (CHE-1048804) and the NIH NCRR (S10RR025631). Calculations were performed on the Hoffman2 cluster and the UCLA Institute of Digital Research and Education (IDRE) at UCLA and the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation (OCI-1053575).
Author information
Authors and Affiliations
Contributions
M.M.Y., A.V.K., M.G. and B.J.S. designed and performed experiments and analysed experimental data. Q.S., B.L. and S.C. designed, performed, and analysed computational data. K.N.H. and N.K.G. directed the investigations and prepared the manuscript with contributions from all authors; all authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Extended data figures and tables
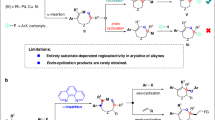
Extended Data Fig. 1 Proposed catalytic cycle.
Computational studies support a mechanism involving activation of the benzotriazinone by the Ni catalyst, migratory insertion across one olefin of the cyclic allene, isomerization to a Ni π-allyl complex, and enantioselective outer-sphere attack to provide the observed phenanthridinone.
Supplementary information
Supplementary Information
This file contains: General Procedures; Reaction Optimization Data; Single Crystal X-Ray Diffraction Data; Supercritical Fluid Chromatography Data; 1H and 13C NMR Spectral Data; Computational Methods; Detailed Computational Mechanistic Analysis, Atomic Coordinates for all Structures Studied Computationally and Supplementary References.
Supplementary Data
This file contains the CheckCIF and CIF file for Compound 69 CCDC reference 1987661.
Rights and permissions
About this article
Cite this article
Yamano, M.M., Kelleghan, A.V., Shao, Q. et al. Intercepting fleeting cyclic allenes with asymmetric nickel catalysis. Nature 586, 242–247 (2020). https://doi.org/10.1038/s41586-020-2701-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2701-2
This article is cited by
-
Rarely used strained molecules step up for organic synthesis
Nature (2023)
-
An enantioselective four-component reaction via assembling two reaction intermediates
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.