Abstract
Gene expression in eukaryotes requires the effective separation of nuclear transcription and RNA processing from cytosolic translation1. This separation is achieved by the nuclear envelope, which controls the exchange of macromolecules through nuclear pores2. During mitosis, however, the nuclear envelope in animal and plant cells disassembles, allowing cytoplasmic and nuclear components to intermix3. When the nuclear envelope is reformed, cytoplasmic components are removed from the nucleus by receptor-mediated transport through nuclear pores2. These pores have a size limit of 39 nanometres4,5,6,7, which raises the question of how larger cytoplasmic molecules are cleared from the nucleus. Here we show in HeLa cells that large cytoplasmic components are displaced before nuclear envelope assembly by the movement of chromosomes to a dense cluster. This clustering occurs when chromosomes approach the poles of anaphase spindles, and is mediated by a microtubule-independent mechanism that involves the surfactant-like protein Ki-67. Ki-67 forms repulsive molecular brushes during the early stages of mitosis8, but during mitotic exit the brushes collapse and Ki-67 promotes chromosome clustering. We show that the exclusion of mature ribosomes from the nucleus after mitosis depends on Ki-67-regulated chromosome clustering. Thus, our study reveals that chromosome mechanics help to re-establish the compartmentalization of eukaryotic cells after open mitosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw microscopy data are available from the corresponding authors upon request, given the large file sizes that are involved. Source data are provided with this paper.
Code availability
All of the steps of the image analyses that were performed in this study are described in detail to allow reproduction of the results. We will provide the code upon request.
References
Maniatis, T. & Reed, R. An extensive network of coupling among gene expression machines. Nature 416, 499–506 (2002).
Görlich, D. & Kutay, U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15, 607–660 (1999).
Ungricht, R. & Kutay, U. Mechanisms and functions of nuclear envelope remodelling. Nat. Rev. Mol. Cell Biol. 18, 229–245 (2017).
Panté, N. & Kann, M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol. Biol. Cell 13, 425–434 (2002).
Bui, K. H. et al. Integrated structural analysis of the human nuclear pore complex scaffold. Cell 155, 1233–1243 (2013).
Eibauer, M. et al. Structure and gating of the nuclear pore complex. Nat. Commun. 6, 7532–7539 (2015).
Beck, M. & Hurt, E. The nuclear pore complex: understanding its function through structural insight. Nat. Rev. Mol. Cell Biol. 18, 73–89 (2017).
Cuylen, S. et al. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature 535, 308–312 (2016).
Delarue, M. et al. mTORC1 controls phase separation and the biophysical properties of the cytoplasm by tuning crowding. Cell 174, 338–3490 (2018).
Deryło, K. et al. The uL10 protein, a component of the ribosomal P-stalk, is released from the ribosome in nucleolar stress. Biochim. Biophys. Acta Mol. Cell Res. 1865, 34–47 (2018).
Görlich, D., Henklein, P., Laskey, R. A. & Hartmann, E. A 41 amino acid motif in importin-α confers binding to importin-β and hence transit into the nucleus. EMBO J. 15, 1810–1817 (1996).
Thomas, F. & Kutay, U. Biogenesis and nuclear export of ribosomal subunits in higher eukaryotes depend on the CRM1 export pathway. J. Cell Sci. 116, 2409–2419 (2003).
Kudo, N. et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl Acad. Sci. USA 96, 9112–9117 (1999).
Potapova, T. A. et al. The reversibility of mitotic exit in vertebrate cells. Nature 440, 954–958 (2006).
Samwer, M. et al. DNA cross-bridging shapes a single nucleus from a set of mitotic chromosomes. Cell 170, 956–972 (2017).
Rosen, M. J. & Kunjappu, J. T. Surfactants and Interfacial Phenomena (John Wiley & Sons, 2012).
Cimini, D., Mattiuzzo, M., Torosantucci, L. & Degrassi, F. Histone hyperacetylation in mitosis prevents sister chromatid separation and produces chromosome segregation defects. Mol. Biol. Cell 14, 3821–3833 (2003).
Booth, D. G. et al. Ki-67 is a PP1-interacting protein that organises the mitotic chromosome periphery. eLife 3, e01641 (2014).
Rivera, V. M. et al. A humanized system for pharmacologic control of gene expression. Nat. Med. 2, 1028–1032 (1996).
Gautier, T., Robert-Nicoud, M., Guilly, M. N. & Hernandez-Verdun, D. Relocation of nucleolar proteins around chromosomes at mitosis. A study by confocal laser scanning microscopy. J. Cell Sci. 102, 729–737 (1992).
Booth, D. G. et al. 3D-CLEM reveals that a major portion of mitotic chromosomes is not chromatin. Mol. Cell 64, 790–802 (2016).
Schmitz, M. H. A. et al. Live-cell imaging RNAi screen identifies PP2A-B55α and importin-β1 as key mitotic exit regulators in human cells. Nat. Cell Biol. 12, 886–893 (2010).
Lukinavičius, G. et al. SiR-Hoechst is a far-red DNA stain for live-cell nanoscopy. Nat. Commun. 6, 8497 (2015).
Ran, F. A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013).
Huang, L.-K. & Wang, M.-J. J. Image thresholding by minimizing the measures of fuzziness. Pattern Recognit. 28, 41–51 (1995).
Sommer, C., Hoefler, R., Samwer, M. & Gerlich, D. W. A deep learning and novelty detection framework for rapid phenotyping in high-content screening. Mol. Biol. Cell 28, 3428–3436 (2017).
Thévenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998).
Tarantino, N. et al. TNF and IL-1 exhibit distinct ubiquitin requirements for inducing NEMO-IKK supramolecular structures. J. Cell Biol. 204, 231–245 (2014).
Acknowledgements
The authors thank S. Tietscher for histone-level verification, K. F. Leesch and the IMBA/IMP/GMI BioOptics and Molecular Biology Service facilities for technical support, M. Tchórzewski for the L10-eGFP plasmid and I. Patten and Life Science Editors (https://www.lifescienceeditors.com) for comments on the manuscript. Research in the laboratory of D.W.G. was supported by the Austrian Academy of Sciences, by an ERC Starting (Consolidator) Grant (number 281198), by the Wiener Wissenschafts-, Forschungs- und Technologiefonds (WWTF; project numbers LS14-009 and LS17-003) and by the Austrian Science Fund (FWF special research program ‘Chromosome Dynamics’ SFB F34-06, Doktoratskolleg ‘Chromosome Dynamics’ DK W1238); research in the laboratory of S.C.-H. was supported by the German Research Foundation (DFG, project number 402723784); M.P., A.H.-A. and M.W.G.S. have received PhD fellowships from the Boehringer Ingelheim Fonds; S.C.-H. has received funding from a Human Frontier Science Program (LTF000996/2013 and CDA00045/2019); and L.J.H. was supported by the National Institutes of Health (R01GM132447) and by the Whitman Fellows programme of the Woods Hole Marine Biology laboratory.
Author information
Authors and Affiliations
Contributions
D.W.G. and S.C.-H. conceived the project. M.P. designed, performed and analysed experiments investigating nucleo-cytoplasmic compartmentalization during anaphase and during chromosome clustering in spindle-less mitotic exit, as well as experiments addressing the role of actin and H3 phosphorylation at Ser10. S.C.-H. designed, performed and analysed experiments addressing the localization, conformation and mechanism of Ki-67 in chromosome clustering, chromosome motility in the clustered state and the inducible H2B–H2B interaction system. A.H.-A. performed and analysed experiments probing chromosome clustering in Ki-67-knockout cells and phenotype rescue by expression of transgenic Ki-67. M.W.G.S. performed initial experiments on GEM localization during mitosis and the effect of trichostatin A on chromosome clustering. M.S. designed, performed and analysed experiments addressing the localization and function of BAF in chromosome clustering, with help from M.P. C.B. characterized the cell lines stably expressing L10–eGFP and GEMs. L.J.H. generated GEM expression constructs. D.W.G. and S.C.-H. acquired funding and supervised the project. D.W.G. and S.C.-H. wrote the manuscript, with help from M.P.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
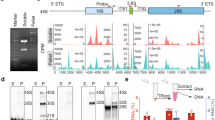
Extended Data Fig. 1 Characterization of GEM- and L10-expressing stable cell lines.
a, Viability of HeLa cell lines stably expressing markers as indicated, measured by counting Hoechst-stained cell nuclei that were negative for the cell death marker TO-PRO-3. Cell numbers: n = 754 (wild-type), n = 930 (GEMs), n = 603 (L10–eGFP). b, Proliferation of HeLa cell lines stably expressing markers as indicated, quantified as fold-change of live cells between 0 and 24 h in time-lapse microscopy videos. Cell number at 24 h: n = 2,001 (wild-type), n = 2,178 (L10–eGFP), n = 2,283 (GEMs). c, Mitotic duration in HeLa cell lines stably expressing markers as indicated, scored by manual measurement of the time from prometaphase to anaphase onset. Cell numbers: n = 320 (wild-type), n = 320 (GEMs), n = 320 (L10–eGFP). d, e, Validation of L10–eGFP incorporation into ribosomes. d, Cell lysate fractions separated on a sucrose gradient. Dashed lines indicate fractions loaded on the gel blotted in e. e, Immunoblot analysis of ribosomal protein L10 in cell lysate fractions, isolated from cells stably expressing L10–eGFP. Annotations of endogenous L10 and L10–eGFP are based on predicted molecular weights. For gel source data, see Supplementary Fig. 1. Bars indicate mean and error bars represent ± s.d.
Extended Data Fig. 2 Ribosomes are excluded from the nucleus in the absence of nuclear envelope transport.
a, HeLa cell expressing the mature ribosome marker L10–eGFP and H2B–mCherry progressing through anaphase in the presence of leptomycin B. b, Quantification of L10–eGFP mean fluorescence intensity within the chromosomal region normalized to surrounding cytoplasm, in anaphase cells as in a. n = 16 cells. c, d, Validation of Exportin-1 inhibition by leptomycin B. c, Live HeLa cells expressing fluorescently labelled H2B and GFP–NES were imaged during mitotic progression by time-lapse microscopy, either untreated (control) or in presence of leptomycin B. Time point 0 min refers to anaphase onset. d, Quantification of GFP–NES mean fluorescence intensity within the chromosomal region normalized to pre-anaphase in control and leptomycin B treated cells, as in c. n = 18 cells (control), n = 15 cells (leptomycin B). Lines indicate mean and shaded areas represent ± s.d. Scale bars, 10 μm.
Extended Data Fig. 3 Chromosomes cluster and the nucleus reassembles in spindle-less cells after the induction of mitotic exit by different experimental procedures.
a–d, Validation of mitotic exit in spindle-less cells by comparing H3(S10) dephosphorylation kinetics during unperturbed anaphase and spindle-less mitotic exit. a, Example images of wild-type HeLa cells during unperturbed anaphase fixed after time-lapse imaging, in metaphase, 6 min (maximally clustered) and 10 min after anaphase onset. Upper panel: chromatin labelled with H2B–mCherry, lower panel: H3(pS10) immunofluorescence. Single z-slice shown. b, Quantification of H3(pS10) mean fluorescence intensity and chromatin area in unperturbed mitosis, as shown in a. Cell numbers: n = 9 (metaphase), n = 14 (3-6 min after anaphase onset), n = 8 (≥8 min after anaphase onset). Normalization to average value of metaphase time point. c, Example images of wild-type HeLa cells during spindle-less mitosis fixed after time-lapse imaging, in prometaphase (no flavopiridol), 10 min (maximally clustered) and 20 min after flavopiridol addition. Imaging as in a. d, Quantification of H3(pS10) mean fluorescence intensity and chromatin area in spindle-less mitosis, as shown in (c), demonstrates that histone 3-serine 10 was efficiently dephosphorylated in flavopiridol-induced mitotic exit and chromosomes cluster to a degree comparable to that of normal late anaphase. Cell numbers: n = 11 (nocodazole arrested pro-metaphase), n = 12 (10 min after flavopiridol addition), n = 13 (20 min after flavopiridol addition). Normalization to average value of prometaphase time point. e, Time-lapse microscopy of HeLa cell expressing IBB–eGFP and H2B–mCherry incubated in nocodazole; flavopiridol was added (t = 0 min) to induce mitotic exit. f, Quantification of IBB–eGFP mean fluorescence intensity within the chromosomal region, normalized to pre-flavopiridol time points, during spindle-less mitotic exit as in e. n = 20 cells. g, Time-lapse microscopy of a HeLa cell expressing H2B–mCherry, progressing through reversine-induced mitotic exit in the absence of spindle. Yellow line indicates convex hull around chromosomes, single z-slice shown. Time is relative to onset of clustering. h, Quantification of chromosome convex hull area of 11 cells as in g. Individual cell curves were aligned based on half-maximum value of convex hull area. Normalization to average of first 4 time points. i, Live HeLa cell undergoing mitosis upon RNAi-mediated depletion of the spindle checkpoint protein MAD2 in the absence of a spindle. The cell line stably expresses H2B–mCherry and membrane marker AcGFP–LAP2β. Time relative to nuclear envelope breakdown (NEBD), single z-slice shown. Representative example of 14 cells shown. Bars indicate mean in b, d; lines and shaded areas indicate mean ± s.d. in f, h. Scale bars, 10 μm.
Extended Data Fig. 4 Chromosome clustering is independent of F-actin, precedes enwrapment by the nuclear envelope and suppresses chromosome mobility.
a, b, Effect of actin depolymerization on chromosome clustering. a, Live HeLa cells stably expressing actin–eGFP and H2B–mCherry were imaged in the absence (control) or presence of latrunculin B. Nocodazole was present in both conditions. b, Quantification of chromosome convex hull area during a flavopiridol induced mitotic exit in the presence of nocodazole, in the absence (control) and presence of latrunculin B as shown in a, normalized to average area pre-flavopiridol addition. Cell numbers: n = 13 (control), n = 22 (latrunculin B). c, Timing of chromosome clustering relative to nuclear envelopment. Live mitotic HeLa cell expressing H2B–mCherry and the inner nuclear envelope protein AcGFP–LAP2β were imaged in presence of nocodazole; flavopiridol was added at t = 0 min to induce mitotic exit. d, Quantification of chromosomal area by convex hull (yellow line in H2B channel in c) and quantification of AcGFP–LAP2β accumulation at the surface of the chromatin region, within a rim of 1.6 μm width (yellow ROI in LAP2β channel in (c)). n = 21 cells. e–h, Kinetochore tracking in cells progressing through spindle-less mitotic exit. e, Live mitotic HeLa cell stably expressing H2B–mCherry and CENP-A–eGFP imaged in the presence of nocodazole before and after flavopiridol addition, time-lapse = 10 s; z-projection. f, Kinetochore trajectories (total length: 240 s) starting 240 s before and at 400 after flavopiridol addition. g, Representative MSD analyses of kinetochore tracks (CENP-A–eGFP) of nocodazole-treated mitotic HeLa cells before and after flavopiridol addition. h, Diffusion coefficients derived from mean square displacement (MSD) analyses of trajectories as in f. Significance was tested by a two-tailed ratio paired t-test (****P = 2.9 × 10−11). n = 13 cells. Bars and lines indicate mean, shaded areas indicate ± s.d., dashed vertical lines refer to flavopiridol addition. Scale bars, 10 μm.
Extended Data Fig. 5 Chromosomes cluster in the presence of a mitotic spindle after flavopiridol-mediated induction of mitotic exit.
Live-cell microscopy of wild-type HeLa cells stably expressing H2B–mCherry synchronized to metaphase by MG132 treatment. a, Upper panel: negative control treated with dimethyl sulfoxide (DMSO) solvent, lower panel: mitotic exit induced through acute flavopiridol addition at t = 0 min. Chromosome arms extend out of the metaphase plate before flavopiridol addition, but densely cluster on the metaphase plate 8 min after flavopiridol addition. b, Quantification of chromosome convex hull area of 24 control and 25 flavopiridol treated cells, as in a. c, Top, acute mitotic spindle depolymerization by addition of nocodazole at t = 2 min leads to chromosome movement out of the metaphase plate; bottom, mitotic exit was induced by flavopiridol (0 min), briefly before nocodazole-mediated spindle disassembly (2 min), preventing movement of chromosomes out of the metaphase plate. d, Chromosome convex hull area measurements of 27 nocodazole and 23 flavopiridol and nocodazole treated cells, as shown in c. Values normalized to average of all frames before first drug treatment. Showing single z-slices, lines and shaded areas indicate mean ± s.d., dashed vertical lines indicate drug addition, scale bars, 10 μm.
Extended Data Fig. 6 Chromosome clustering is not mediated by association with BAF or removal of Ki-67.
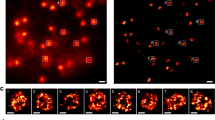
a–c, Effect of BAF depletion on chromosome clustering. a, Live mitotic HeLa cells stably expressing H2B–mCherry and GEMs were imaged 72 h after siRNA transfection, in the presence of nocodazole; flavopiridol was added at t = 0 min to induce mitotic exit. White dashed lines indicate chromosomal areas, single z-slice shown. b, Quantification of chromosome convex hull area, normalized to pre-flavopiridol addition. c, GEM particle count within chromosomal area normalized to pre-flavopiridol addition. n = 23 cells (siBAF), n = 23 cells (siControl). d, Immunoblot analysis of BAF and actin 72 h after siRNA transfection, showing one of two biological replicates. For gel source data, see Supplementary Fig. 1. e, Localization of BAF–eGFP in live mitotic HeLa cell imaged in the presence of nocodazole; flavopiridol was added at t = 0 s to induce mitotic exit. f, Quantification of chromosome convex hull area and BAF–eGFP accumulation at the surface of the chromatin region as in e, normalized to pre-flavopiridol. n = 21 cells. g, Localization of Ki-67, eGFP tagged on endogenous loci in live HeLa cell progressing from metaphase to anaphase (anaphase onset = 0 min), DNA was stained with SiR-Hoechst, z-projection. h, Quantification of chromosome convex hull area and eGFP–Ki-67 mean fluorescence intensity on chromosomes, normalized to pre-anaphase, for 41 cells as in g. i, Localization of Ki-67 during spindle-less mitotic exit. Time-lapse microscopy of mitotic HeLa cell as in g, in the presence of nocodazole; flavopiridol was added (t = 0 min) to induce mitotic exit. Z-projection. j, Quantification of chromosome convex hull area and eGFP–Ki-67 mean fluorescence intensity on chromosomes of 27 cells as in i. Lines and shaded areas indicate mean ± s.d., scale bars, 10 μm.
Extended Data Fig. 7 Ki-67 is required for chromosome clustering in Ki-67-knockout cells after histone overexpression or histone hyperacetylation.
a, Time-lapse microscopy of clustering-deficient HeLa Ki-67-KO cell stably expressing H2B–mCherry to high levels during a flavopiridol-induced mitotic exit in the presence of nocodazole. Transient expression of eGFP–Ki-67 to levels at least matching Ki-67 endogenous levels (lower panel). Single z-slice shown. b, Chromosome convex hull area quantification, normalized to pre-flavopiridol time points, of 29 Ki-67-KO and 28 Ki-67-KO + eGFP–Ki-67cells as in a. c, Immunoblot analysis of H2B from cell lysates, isolated from wild-type cells (1) or cells transiently expressing H2B–mNeonGreen (2, 3). Sample 3 was lysed after 48 h, whereas sample 2 was additionally FACS sorted for the 10% brightest cells which we estimated to represent the population of cells that suppress the Ki-67-knockout individualization failure phenotype. For gel source data, see Supplementary Fig. 1. n = 2 biological repeats. d, e, Immunofluorescence of acetylated H3 of Ki-67-KO cells arrested in nocodazole with or without trichostatin A. d, Representative examples. Single z-slices shown. e, Quantification of acetylated H3 mean fluorescence intensity in Ki-67-KO cells arrested in nocodazole with and without trichostatin A. Bars represent mean, significance was tested with a two-tailed Kolmogorov–Smirnov test. (****P = 2.22 × 10−16). n = 115 cells (Ki-67-KO), n = 111 cells (Ki-67-KO + TSA). f, Time-lapse microscopy of HeLa wild-type or clustering-deficient Ki-67-KO cells with or without transient re-expression of Ki-67 during a flavopiridol-induced mitotic exit in the presence of nocodazole. Cells were treated with trichostatin A 2 h before imaging to rescue the Ki-67-knockout individualization failure phenotype in Ki-67-KO cells. Representative examples stained with SiR-Hoechst, single z-slices shown. g, Chromosome convex hull area, normalized to pre-flavopiridol time points, quantification of 21 Ki-67-KO, 31 wild-type and 32 Ki-67-KO + eGFP–Ki-67 cells as in f. Lines and shaded areas indicate mean ± s.d. (b, g), scale bars, 10 μm.
Extended Data Fig. 8 Ki-67 does not mediate chromosome clustering through PP1 recruitment or through H3(S10) dephosphorylation.
a, Schematic of genotyping strategy to endogenously mutate Ki-67’s PP1-binding motif RVSF to RASA. A newly generated SacII restriction site generated by CRISPR–Cas9 nickase as depicted was used to detect correctly mutated alleles. b, SacII restriction fragments were detected by gel electrophoresis following the assay depicted in a, showing successful recombination of all three Ki-67 alleles present in HeLa cells for clone 43 and 96. Showing one example of two biological replicates. c, d, Spindle-less mitotic exit in wild-type cells and in homozygous Ki-67(RASA)-mutant cells. Live cells were imaged in the presence of nocodazole; flavopiridol was added (t = 0 min) to induce mitotic exit. Quantification of chromosome convex hull area, normalized to pre-flavopiridol time points (c) and representative examples stained with SiR-Hoechst (d). Z-projection. Lines and shaded areas indicate mean ± s.d. n = 22 cells (wild-type), n = 23 cells (RASA). e, Molecular organization of the Ki-67 RASA mutant on the surface of mitotic chromosomes before and after flavopiridol addition to taxol treated cells. Ki-67(RASA) was tagged by mCherry and eGFP on either protein end, respectively, and expressed in HeLa cells bearing the endogenous RASA mutation in all three copies of Ki-67. Bars represent mean, significance was tested by a two-tailed unpaired t-test (****P = 8.3 × 10−8, ***P = 0.00049). Chromosome numbers: n = 32 (R-Ki-67-G, pre), n = 28 (R-Ki-67-G, post), n = 37 (G-Ki-67-R, pre), n = 34 (G-Ki-67-R, post). f, g, Immunofluorescence of H3(pS10) during spindle-less mitotic exit in wild-type and Ki-67-KO cells. f, Representative examples of wild-type and Ki-67-KO cells before, 10 and 20 min after flavopiridol addition. Single z-slice is shown. g, Quantification of H3(pS10) mean fluorescence intensity before (wild-type n = 38 cells, Ki-67-KO n = 23 cells) and 5 min (wild-type n = 61 cells, Ki-67-KO n = 61 cells), 10 min (wild-type n = 72 cells, Ki-67-KO n = 65 cells) and 20 min (wild-type n = 73 cells, Ki-67-KO n = 55 cells) after mitotic exit induction with flavopiridol in wild-type and Ki-67-KO cells. Values normalized to average of wild-type 5 min time point. Showing combined data of two independent biological replicates. Bars represent mean, significance was tested with a two-tailed Mann–Whitney test (P = 0.72 for pre-flavopiridol time point, P = 0.96 for 5 min time point, P = 0.71 for 10 min time point and P = 0.26 for 20 min time point). Scale bars, 10 μm.
Extended Data Fig. 9 Chromosome clustering can be artificially induced through increased adhesion between chromosomes.
a, Design of an inducible H2B–H2B interaction system. b, Live HeLa cells expressing H2B–mCherry–FKBP and H2B–mNeonGreen–FRB or only H2B–mCherry–FKBP were imaged before and after rapamycin addition. Z-projection; scale bar, 10 μm. c, Normalized chromosome convex hull area quantification as in b of 23 cells coexpressing H2B–mCherry–FKBP and H2B–mNeonGreen–FRB, versus 12 negative control cells expressing H2B–mCherry–FKBP alone. Lines and shaded areas indicate mean ± s.d.
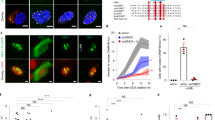
Extended Data Fig. 10 Clustering-deficient cells assemble a transport-competent nuclear envelope.
a, Time-lapse microscopy of clustering-deficient HeLa Ki-67-KO cells transiently expressing H2B–mCherry to high levels, and stably expressing IBB–eGFP, during a flavopiridol-induced mitotic exit in the presence of nocodazole. b, Quantification of IBB–eGFP mean fluorescence intensity within the chromosomal area (green) and quantification of chromosome convex hull area (magenta) in clustering-deficient cells as in a, normalized to pre-flavopiridol. n = 15 cells. c, d, H2B–mCherry expression levels and chromosomal area measurements for cells shown in Fig. 4a–c. c, Quantification of H2B–mCherry total fluorescence for cells analysed in Fig. 4c (all data points normalized to mean of wild-type dataset). d, Chromosome convex hull area quantification (normalized to pre-flavopiridol area) for cells quantified in Fig. 4c. n = 46 cells (wild-type), n = 29 cells (Ki-67-KO). e, Clustering-proficient HeLa cell imaged as in Fig. 4d, but treated with leptomycin B. f, Quantification of total L10 fluorescence in nuclear regions as shown in e, normalized to average of pre-flavopiridol addition. n = 22. g, h, Quantification of H2B–mCherry total fluorescence and chromosome convex hull area for cells analysed in Fig. 4f and in f. Normalizations as in c, d, cell numbers: n = 23 wild-type, n = 8 Ki-67-KO, n = 22 leptomycin B. Individual Ki-67-KO curves shown in Supplementary Fig. 2. Significance was tested with two-sided unpaired t-test (P = 0.28 in GEM cell lines wild-type versus Ki-67-KO, P = 0.12 in L10 cell lines wild-type versus LMB-treated and P = 0.13 in L10 cell lines wild-type versus Ki-67-KO). Lines and shaded areas indicate mean ± s.d., dashed vertical lines indicate flavopiridol addition. Scale bars, 10 μm. Single z-slices are shown.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-2 and Supplementary Tables 1-2.
Video 1
GEM exclusion during anaphase Airyscan imaging of live HeLa cell expressing GEMs (green) and H2B-mCherry (magenta) progressing through anaphase. Relates to data shown in Fig. 1d, e. Scale bar, 10 μm. Cell number, n = 22.
Video 2
Mature ribosome exclusion during anaphase Confocal imaging of live HeLa cell expressing L10-EGFP (green) and H2B-mCherry (magenta) progressing through anaphase. Relates to data shown in Fig. 1f, g. Scale bar, 10 μm. Cell number, n = 30.
Video 3
Chromosome motility during early mitosis and during mitotic exit in the clustered state. Live mitotic HeLa cell stably expressing H2B-mCherry (magenta) and CENP-A-EGFP (green) was imaged in the presence of nocodazole before (left panel) and after flavopiridol addition (right panel), time relative to flavopiridol addition. Relates to data shown in Extended Data Fig. 4e, f. Scale bar, 10 μm. n = 13.
Video 4
Chromosome clustering by an inducible H2B-H2B interaction system Live mitotic HeLa cell transiently expressing H2B-mCherry-FKBP and H2B-mNeonGreen-FRB (left cell) or H2B-mCherry-FKBP alone (right cell) was imaged in the presence of nocodazole before and after rapamycin addition, time relative to rapamycin addition, H2B-mCherry-FKBP fluorescence shown. Relates to data shown in Extended Data Fig. 9b, c. Scale bar, 10 μm. 3 independent experiments were performed with similar results with a total cell number of 23 cells co-expressing H2B-mCherry-FKBP and H2B-mNeonGreen-FRB and 12 negative control cells expressing H2B-mCherry-FKBP alone.
Video 5
Exclusion of GEMs during nuclear assembly depends on Ki-67-regulated chromosome clustering Timelapse microscopy of spindle-less mitotic exit in clustering proficient wild-type HeLa cell (upper panel) and clustering deficient Ki-67 KO HeLa cell (lower panel). Cells stably expressing GEMs (green) and transiently expressing H2B-mCherry (magenta); time relative to flavopiridol addition. Relates to data shown in Fig. 4a-c. Scale bar, 10 μm. Cell number, n = 46 wild-type and n = 29 Ki-67 KO.
Source data
Rights and permissions
About this article
Cite this article
Cuylen-Haering, S., Petrovic, M., Hernandez-Armendariz, A. et al. Chromosome clustering by Ki-67 excludes cytoplasm during nuclear assembly. Nature 587, 285–290 (2020). https://doi.org/10.1038/s41586-020-2672-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2672-3
This article is cited by
-
Determinants that enable disordered protein assembly into discrete condensed phases
Nature Chemistry (2024)
-
A high-content screen reveals new regulators of nuclear membrane stability
Scientific Reports (2024)
-
The molecular basis for cellular function of intrinsically disordered protein regions
Nature Reviews Molecular Cell Biology (2024)
-
Temporal and spatial topography of cell proliferation in cancer
Nature Cell Biology (2022)
-
Protein-Peptide Turnover Profiling reveals the order of PTM addition and removal during protein maturation
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.