Abstract
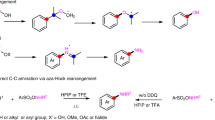
Chemical reactions that reliably join two molecular fragments together (cross-couplings) are essential to the discovery and manufacture of pharmaceuticals and agrochemicals1,2. The introduction of amines onto functionalized aromatics at specific and pre-determined positions (ortho versus meta versus para) is currently achievable only in transition-metal-catalysed processes and requires halogen- or boron-containing substrates3,4,5,6. The introduction of these groups around the aromatic unit is dictated by the intrinsic reactivity profile of the method (electrophilic halogenation or C–H borylation) so selective targeting of all positions is often not possible. Here we report a non-canonical cross-coupling approach for the construction of anilines, exploiting saturated cyclohexanones as aryl electrophile surrogates. Condensation between amines and carbonyls, a process that frequently occurs in nature and is often used by (bio-)organic chemists7, enables a predetermined and site-selective carbon–nitrogen (C–N) bond formation, while a photoredox- and cobalt-based catalytic system progressively desaturates the cyclohexene ring en route to the aniline. Given that functionalized cyclohexanones are readily accessible with complete regiocontrol using the well established carbonyl reactivity, this approach bypasses some of the frequent selectivity issues of aromatic chemistry. We demonstrate the utility of this C–N coupling protocol by preparing commercial medicines and by the late-stage amination–aromatization of natural products, steroids and terpene feedstocks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Materials and methods, experimental procedures, useful information, mechanistic studies, optimization studies, 1H NMR spectra, 13C NMR spectra and mass spectrometry data are available in the Supplementary Information. Raw data are available from the corresponding author on reasonable request.
References
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Campos, K. R. et al. The importance of synthetic chemistry in the pharmaceutical industry. Science 363, eaat0805 (2019).
Hartwig, J. F. Carbon–heteroatom bond-forming reductive eliminations of amines, ethers, and sulfides. Acc. Chem. Res. 31, 852–860 (1998).
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
West, M. J., Fyfe, J. W. B., Vantourout, J. C. & Watson, A. J. B. Mechanistic development and recent applications of the Chan–Lam amination. Chem. Rev. 119, 12491–12523 (2019).
Beletskaya, I. P. & Cheprakov, V. A. Copper in cross-coupling reactions: the post-Ullmann chemistry. Coord. Chem. Rev. 248, 2337–2364 (2004).
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
Crawley, M. L. & Trost, B. M. Applications Of Transition Metal Catalysis In Drug Discovery And Development: An Industrial Perspective (John Wiley & Sons, 2012).
Hartwig, J. F. Carbon–heteroatom bond formation catalyzed by organometallic complexes. Nature 455, 314–322 (2008).
Olah, G. A. Friedel-Crafts And Related Reactions (Wiley, 1963).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C-H activation for the construction of C-B bonds. Chem. Rev. 110, 890–931 (2010).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C-H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Ruffoni, A. et al. Practical and regioselective amination of arenes using alkylamines. Nat. Chem. 11, 426–433 (2019).
Boursalian, G. B., Ham, W. S., Mazzotti, A. R. & Ritter, T. Charge-transfer-directed radical substitution enables para-selective C–H functionalization. Nat. Chem. 8, 810–815 (2016).
Johnsson, K., Allemann, R. K., Widmer, H. & Benner, S. A. Synthesis, structure and activity of artificial, rationally designed catalytic polypeptides. Nature 365, 530–532 (1993).
Grondal, C., Jeanty, M. & Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem. 2, 167–178 (2010).
Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 554, 41–49 (2018).
Izawa, Y., Pun, D. & Stahl, S. S. Palladium-catalyzed aerobic dehydrogenation of substituted cyclohexanones to phenols. Science 333, 209–213 (2011).
Koizumi, Y. et al. Selective synthesis of primary anilines from NH3 and cyclohexanones by utilizing preferential adsorption of styrene on the Pd nanoparticle surface. Angew. Chem. Int. Ed. 58, 10893–10897 (2019).
Hong, W. P., Iosub, A. V. & Stahl, S. S. Pd-catalyzed Semmler–Wolff reactions for the conversion of substituted cyclohexenone oximes to primary anilines. J. Am. Chem. Soc. 135, 13664–13667 (2013).
Li, Y., Wang, D., Zhang, L. & Luo, S. Redox property of enamines. J. Org. Chem. 84, 12071–12090 (2019).
Pirnot, M. T., Rankic, D. A., Martin, D. B. C. & MacMillan, D. W. C. Photoredox activation for the direct β-arylation of ketones and aldehydes. Science 339, 1593–1596 (2013).
West, J. G., Huang, D. & Sorensen, E. J. Acceptorless dehydrogenation of small molecules through cooperative base metal catalysis. Nat. Commun. 6, 10093 (2015).
Sun, X., Chen, J. & Ritter, T. Catalytic dehydrogenative decarboxyolefination of carboxylic acids. Nat. Chem. 10, 1229–1233 (2018).
Weiss, M. E., Kreis, L. M., Lauber, A. & Carreira, E. M. Cobalt-catalyzed coupling of alkyl iodides with alkenes: deprotonation of hydridocobalt enables turnover. Angew. Chem. Int. Ed. 50, 11125–11128 (2011).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Nicholas, A. M. P. & Arnold, D. R. Thermochemical parameters for organic radicals and radical ions. Part 1. The estimation of the pKa of radical cations based on thermochemical calculations. Can. J. Chem. 60, 2165–2179 (1982).
Gridnev, A. A. & Ittel, S. D. Catalytic chain transfer in free-radical polymerizations. Chem. Rev. 101, 3611–3660 (2001).
Dempsey, J. L., Brunschwig, B. S., Winkler, J. R. & Gray, H. B. Hydrogen evolution catalyzed by cobaloximes. Acc. Chem. Res. 42, 1995–2004 (2009).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Santanilla, A. B. et al. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 347, 49–53 (2015).
Park, N. H., Vinogradova, E. V., Surry, D. S. & Buchwald, S. L. Design of new ligands for the palladium-catalyzed arylation of α-branched secondary amines. Angew. Chem. Int. Ed. 54, 8259–8262 (2015).
Morris, S. A., Wang, J. & Zheng, N. The prowess of photogenerated amine radical cations in cascade reactions: from carbocycles to heterocycles. Acc. Chem. Res. 49, 1957–1968 (2016).
Brusoe, A. T. & Hartwig, J. F. Palladium-catalyzed arylation of fluoroalkylamines. J. Am. Chem. Soc. 137, 8460–8468 (2015).
Dobrikov, G. M. et al. Synthesis and anti-enterovirus activity of new analogues of MDL-860. Bioorg. Med. Chem. Lett. 27, 4540–4543 (2017).
Sulur, M. et al. Development of scalable manufacturing routes to AZD1981. Application of the Semmler–Wolff aromatisation for synthesis of the indole-4-amide core. Org. Process Res. Dev. 16, 1746–1753 (2012).
Zhao, Y., Yim, W.-L., Tan, C. K. & Yeung, Y.-Y. An unexpected oxidation of unactivated methylene C–H using DIB/TBHP protocol. Org. Lett. 13, 4308–4311 (2011).
Cooke, J., Glover, B. N., Lawrence, R. M., Sharp, M. J. & Tymoschenko, M. F. WO 02/066418 (Glaxo-SmithKline, 2002).
See, Y. Y., Dang, T. T., Chen, A. & Seayad, A. M. Concise synthesis of vesnarinone and its analogues by using Pd-catalyzed C–N bond-forming reactions. Eur. J. Org. Chem. 2014, 7405–7412 (2014).
Acknowledgements
D.L. thanks EPSRC for a Fellowship (EP/P004997/1) and the European Research Council for a research grant (758427). S.U.D. thanks the Marie Curie Actions for a Fellowship (791349).
Author information
Authors and Affiliations
Contributions
S.U.D. and D.L. designed the project. S.U.D., F.J. and A.L. performed all experiments. All the authors analysed the results. D.L., F.J. and J.J.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Edward Anderson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
The Supplementary Information file contains the following sections: (1) general experimental details; (2) starting material synthesis; (3) reaction optimization; (4) pictures of reaction set-up; (5) substrate scope; (6) mechanistic considerations; (7) NMR spectra; and (8) references.
Rights and permissions
About this article
Cite this article
U. Dighe, S., Juliá, F., Luridiana, A. et al. A photochemical dehydrogenative strategy for aniline synthesis. Nature 584, 75–81 (2020). https://doi.org/10.1038/s41586-020-2539-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2539-7
This article is cited by
-
Chalcogen-bridged coordination polymer for the photocatalytic activation of aryl halides
Nature Communications (2023)
-
Decarboxylative tandem C-N coupling with nitroarenes via SH2 mechanism
Nature Communications (2022)
-
Bioinspired desaturation of alcohols enabled by photoredox proton-coupled electron transfer and cobalt dual catalysis
Nature Communications (2022)
-
A cross-dehydrogenative C(sp3)−H heteroarylation via photo-induced catalytic chlorine radical generation
Nature Communications (2021)
-
Hydroxylamine-mediated C–C amination via an aza-hock rearrangement
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.