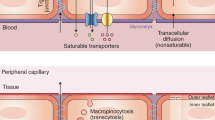
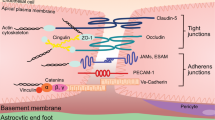
Abstract
The vascular interface of the brain, known as the blood–brain barrier (BBB), is understood to maintain brain function in part via its low transcellular permeability1,2,3. Yet, recent studies have demonstrated that brain ageing is sensitive to circulatory proteins4,5. Thus, it is unclear whether permeability to individually injected exogenous tracers—as is standard in BBB studies—fully represents blood-to-brain transport. Here we label hundreds of proteins constituting the mouse blood plasma proteome, and upon their systemic administration, study the BBB with its physiological ligand. We find that plasma proteins readily permeate the healthy brain parenchyma, with transport maintained by BBB-specific transcriptional programmes. Unlike IgG antibody, plasma protein uptake diminishes in the aged brain, driven by an age-related shift in transport from ligand-specific receptor-mediated to non-specific caveolar transcytosis. This age-related shift occurs alongside a specific loss of pericyte coverage. Pharmacological inhibition of the age-upregulated phosphatase ALPL, a predicted negative regulator of transport, enhances brain uptake of therapeutically relevant transferrin, transferrin receptor antibody and plasma. These findings reveal the extent of physiological protein transcytosis to the healthy brain, a mechanism of widespread BBB dysfunction with age and a strategy for enhanced drug delivery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw sequencing data are deposited under NCBI GEO: GSE134058 and GSE142500. Raw lipidomics data are available from figshare (https://doi.org/10.6084/m9.figshare.6025748). Source data are provided with this paper.
References
Obermeier, B., Daneman, R. & Ransohoff, R. M. Development, maintenance and disruption of the blood–brain barrier. Nat. Med. 19, 1584–1596 (2013).
Chow, B. W. & Gu, C. The molecular constituents of the blood–brain barrier. Trends Neurosci. 38, 598–608 (2015).
Profaci, C. P., Munji, R. N., Pulido, R. S. & Daneman, R. The blood–brain barrier in health and disease: important unanswered questions. J. Exp. Med. 217, e20190062 (2020).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011).
Saunders, N. R., Daneman, R., Dziegielewska, K. M. & Liddelow, S. A. Transporters of the blood–brain and blood–CSF interfaces in development and in the adult. Mol. Aspects Med. 34, 742–752 (2013).
Saunders, N. R. et al. The rights and wrongs of blood–brain barrier permeability studies: a walk through 100 years of history. Front. Neurosci. 8, 404 (2014).
Reese, T. S. & Karnovsky, M. J. Fine structural localization of a blood–brain barrier to exogenous peroxidase. J. Cell Biol. 34, 207–217 (1967).
Poduslo, J. F., Curran, G. L. & Berg, C. T. Macromolecular permeability across the blood–nerve and blood–brain barriers. Proc. Natl Acad. Sci. USA 91, 5705–5709 (1994).
Yu, Y. J. & Watts, R. J. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics 10, 459–472 (2013).
Zuchero, Y. J. Y. et al. Discovery of novel blood–brain barrier targets to enhance brain uptake of therapeutic antibodies. Neuron 89, 70–82 (2016).
Niewoehner, J. et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 81, 49–60 (2014).
Bell, R. D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012).
Ben-Zvi, A. et al. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 509, 507–511 (2014).
Daneman, R. et al. The mouse blood–brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS ONE 5, e13741 (2010).
Vanlandewijck, M. et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480 (2018).
Montagne, A. et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Yousef, H. et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 25, 988–1000 (2019).
Andreone, B. J. et al. Blood–brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 94, 581–594.e5 (2017).
Armulik, A. et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010).
Keller, A. et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat. Genet. 45, 1077–1082 (2013).
Zarb, Y. et al. Ossified blood vessels in primary familial brain calcification elicit a neurotoxic astrocyte response. Brain 142, 885–902 (2019).
Murshed, M., Harmey, D., Millán, J. L., McKee, M. D. & Karsenty, G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 19, 1093–1104 (2005).
Dahl, R. et al. Discovery and validation of a series of aryl sulfonamides as selective inhibitors of tissue-nonspecific alkaline phosphatase (TNAP). J. Med. Chem. 52, 6919–6925 (2009).
Montagne, A., Zhao, Z. & Zlokovic, B. V. Alzheimer’s disease: a matter of blood–brain barrier dysfunction? J. Exp. Med. 214, 3151–3169 (2017).
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011).
Petersen, M. A., Ryu, J. K. & Akassoglou, K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat. Rev. Neurosci. 19, 283–301 (2018).
Erdő, F., Denes, L. & de Lange, E. Age-associated physiological and pathological changes at the blood–brain barrier: a review. J. Cereb. Blood Flow Metab. 37, 4–24 (2017).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Hall, C. N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014).
Vardy, E. R. L. C., Kellett, K. A. B., Cocklin, S. L. & Hooper, N. M. Alkaline phosphatase is increased in both brain and plasma in Alzheimer’s disease. Neurodegener. Dis. 9, 31–37 (2012).
Zlokovic, B. V., Deane, R., Sagare, A. P., Bell, R. D. & Winkler, E. A. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J. Neurochem. 115, 1077–1089 (2010).
Yang, A. C. et al. Multiple click-selective tRNA synthetases expand mammalian cell-specific proteomics. J. Am. Chem. Soc. 140, 7046–7051 (2018).
Hughes, C. S. et al. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 10, 757 (2014).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 11, 2301–2319 (2016).
Castellano, J. M. et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 544, 488–492 (2017).
Treweek, J. B. et al. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat. Protoc. 10, 1860–1896 (2015).
Tournoux, F. et al. Validation of noninvasive measurements of cardiac output in mice using echocardiography. J. Am. Soc. Echocardiogr. 24, 465–470 (2011).
Domínguez, E. et al. Non-invasive in vivo measurement of cardiac output in C57BL/6 mice using high frequency transthoracic ultrasound: evaluation of gender and body weight effects. Int. J. Cardiovasc. Imaging 30, 1237–1244 (2014).
Villeda, S. A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663 (2014).
Schnell, S. A., Staines, W. A. & Wessendorf, M. W. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J. Histochem. Cytochem. 47, 719–730 (1999).
Triguero, D., Buciak, J. & Pardridge, W. M. Capillary depletion method for quantification of blood–brain barrier transport of circulating peptides and plasma proteins. J. Neurochem. 54, 1882–1888 (1990).
Singh, I. et al. Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance. Proc. Natl Acad. Sci. USA 110, 14771–14776 (2013).
Paris-Robidas, S., Brouard, D., Emond, V., Parent, M. & Calon, F. Internalization of targeted quantum dots by brain capillary endothelial cells in vivo. J. Cereb. Blood Flow Metab. 36, 731–742 (2016).
Preston, J. E., al-Sarraf, H. & Segal, M. B. Permeability of the developing blood–brain barrier to 14C-mannitol using the rat in situ brain perfusion technique. Brain Res. Dev. Brain Res. 87, 69–76 (1995).
Dan, M., Cochran, D. B., Yokel, R. A. & Dziubla, T. D. Binding, transcytosis and biodistribution of anti-PECAM-1 iron oxide nanoparticles for brain-targeted delivery. PLoS ONE 8, e81051 (2013).
Boulay, A. C., Saubaméa, B., Declèves, X. & Cohen-Salmon, M. Purification of mouse brain vessels. J. Vis. Exp. 105, e53208 (2015).
Ferreira, C. L. et al. Comparison of bifunctional chelates for 64Cu antibody imaging. Eur. J. Nucl. Med. Mol. Imaging 37, 2117–2126 (2010).
Ilovich, O. et al. Development and validation of an immuno-PET tracer as a companion diagnostic agent for antibody–drug conjugate therapy to target the CA6 epitope. Radiology 276, 191–198 (2015).
Chaney, A. et al. 11C-DPA-713 versus 18F-GE-180: a preclinical comparison of translocator protein 18 kDa PET tracers to visualize acute and chronic neuroinflammation in a mouse model of ischemic stroke. J. Nucl. Med. 60, 122–128 (2018).
Chaney, A. M., Johnson, E. M., Cropper, H. C. & James, M. L. PET imaging of neuroinflammation using [11C]DPA-713 in a mouse model of ischemic stroke. J. Vis. Exp. 136, 57243 (2018).
James, M. L. et al. New positron emission tomography (PET) radioligand for imaging σ-1 receptors in living subjects. J. Med. Chem. 55, 8272–8282 (2012).
Tabula Muris Consortium. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Wu, A. R. et al. Quantitative assessment of single-cell RNA-sequencing methods. Nat. Methods 11, 41–46 (2014).
Chen, M. B. et al. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep. 30, 4418–4432.e4 (2020).
Li, Q. et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207–223.e10 (2019).
Tsafrir, D. et al. Sorting points into neighborhoods (SPIN): data analysis and visualization by ordering distance matrices. Bioinformatics 21, 2301–2308 (2005).
Yanagida, K. et al. Size-selective opening of the blood–brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc. Natl Acad. Sci. USA 114, 4531–4536 (2017).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Pluvinage, J. V. et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 568, 187–192 (2019).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012).
Xavier, A. L. R. et al. Cannula implantation into the cisterna magna of rodents. J. Vis. Exp. 135, 57378 (2018).
Renier, N. et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014).
Cajka, T., Smilowitz, J. T. & Fiehn, O. Validating quantitative untargeted lipidomics across nine liquid chromatography-high-resolution mass spectrometry platforms. Anal. Chem. 89, 12360–12368 (2017).
Contrepois, K. et al. Cross-platform comparison of untargeted and targeted lipidomics approaches on aging mouse plasma. Sci. Rep. 8, 17747 (2018).
Zemski Berry, K. A., Murphy, R. C., Kosmider, B. & Mason, R. J. Lipidomic characterization and localization of phospholipids in the human lung. J. Lipid Res. 58, 926–933 (2017).
Luo, J. et al. Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front. Neurol. 5, 12 (2014).
Kadakkuzha, B. M. et al. Transcriptome analyses of adult mouse brain reveal enrichment of lncRNAs in specific brain regions and neuronal populations. Front. Cell. Neurosci. 9, 63 (2015).
Sharma, K. et al. Cell type- and brain region-resolved mouse brain proteome. Nat. Neurosci. 18, 1819–1831 (2015).
Dougherty, J. D., Schmidt, E. F., Nakajima, M. & Heintz, N. Analytical approaches to RNA profiling data for the identification of genes enriched in specific cells. Nucleic Acids Res. 38, 4218–4230 (2010).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Harris, M. A. et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 32, D258–D261 (2004).
Zhang, X. D. et al. Traumatic brain injury imaging in the second near-infrared window with a molecular fluorophore. Adv. Mater. 28, 6872–6879 (2016).
Kiick, K. L., Saxon, E., Tirrell, D. A. & Bertozzi, C. R. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc. Natl Acad. Sci. USA 99, 19–24 (2002).
Calve, S., Witten, A. J., Ocken, A. R. & Kinzer-Ursem, T. L. Incorporation of non-canonical amino acids into the developing murine proteome. Sci. Rep. 6, 32377 (2016).
Liu, A. P., Aguet, F., Danuser, G. & Schmid, S. L. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J. Cell Biol. 191, 1381–1393 (2010).
Baruch, K. et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346, 89–93 (2014).
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Fuchs, S. B. A. et al. GeneAnalytics: an integrative gene set analysis tool for next generation sequencing, RNAseq and microarray data. OMICS 20, 139–151 (2016).
Eden, E., Navon, R., Steinfeld, I., Lipson, D. & Yakhini, Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10, 48 (2009).
Saunders, N. R., Dziegielewska, K. M., Møllgård, K. & Habgood, M. D. Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain. J. Physiol. (Lond.) 596, 5723–5756 (2018).
Zhao, Z., Nelson, A. R., Betsholtz, C. & Zlokovic, B. V. Establishment and dysfunction of the blood–brain barrier. Cell 163, 1064–1078 (2015).
Ximerakis, M. et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat. Neurosci. 22, 1696–1708 (2019).
Simionescu, M. et al. The cerebral microvasculature of the rat: structure and luminal surface properties during early development. J. Submicrosc. Cytol. Pathol. 20, 243–261 (1988).
Hervé, F., Ghinea, N. & Scherrmann, J.-M. CNS delivery via adsorptive transcytosis. AAPS J. 10, 455–472 (2008).
Sabbagh, M. F. et al. Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. eLife 7, e36187 (2018).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53 (2016).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, eaal3321 (2017).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Harold, D. et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 41, 1088–1093 (2009).
Acknowledgements
We thank K. Brewer, O. Leventhal, N. Schaum, S. R. Shuken, C. Munson, J. V. Pluvinage, C. A. Maat, N. Khoury and other members of the Wyss-Coray, James, Snyder and Elias laboratories for feedback and support; H. Zhang and K. Dickey for laboratory management; B. Carter for flow cytometry technical expertise; J. Mulholland and Y. Lim of the Stanford Cell Sciences Imaging Facility for help with high-resolution microscopy; and U. Langen and C. Gu for the MFSD2A antibody. This work was funded by the Department of Veterans Affairs (to T.W.-C.), the National Institute on Aging (DP1-AG053015 to T.W.-C., T32-AG0047126 to A.C.Y. and 1RF1AG059694 to T.W.-C. and J.L.), the National Institute of General Medical Sciences (R01-GM059907 to C.R.B.), the NOMIS Foundation (to T.W.-C.), the Glenn Foundation for Aging Research (to T.W.-C.), the National Institutes of Health (3P50HG00773505S1 to M.P.S.), and the Big Idea Brain Rejuvenation Project from the Wu Tsai Neurosciences Institute (to T.W.-C., J.E.E. and C.R.B.). A.C.Y was supported by a Siebel Scholarship. M.Y.S. is a Wallenberg Foundation postdoctoral fellow. S.R.Q. is a Chan Zuckerberg Investigator. We acknowledge the National Institutes of Health shared equipment grants, 1S10OD025091-01 and 1S10OD01227601 from the National Center for Research Resources (NCRR). This study was supported by the AHA-Allen Initiative in Brain Health and Cognitive Impairment: 19PABHI34580007. The statements in this work are solely the responsibility of the authors and do not necessarily represent the views of the American Heart Association (AHA) or the Paul G. Allen Frontiers Group.
Author information
Authors and Affiliations
Contributions
A.C.Y. and T.W.-C. conceptualized the study. A.C.Y. devised and performed plasma labelling experiments. A.C.Y., M.Y.S., L.Z., N.O., D.B., J.E.E. and C.R.B. characterized plasma labelling. D.S., D.P.L., R.H., H.d.B., L.L., J.L., E.Y.W. and A.C.Y. performed and analysed histology experiments. M.Y.S., A.C., H.C.C., A.C.Y. and M.L.J. designed and performed radiotracing experiments. M.B.C., B.L., R.T.V., W.C., A.C.Y. and S.R.Q. performed and analysed RNA-seq experiments. K.C., A.C.Y., G.M.T. and M.P.S. performed and analysed mass spectrometry lipidomics experiments. L.Z., A.C.Y., W.C. and J.E.E. performed and analysed mass spectrometry proteomics experiments. D.G. and A.C.Y. performed and analysed flow cytometry experiments. T.I. and A.C.Y. performed plasma co-injections into the CSF and blood. W.C. and A.C.Y. performed denaturing gel experiments. A.C.Y. wrote the manuscript. M.B.C., M.Y.S., D.S., K.C., L.Z., N.O., B.L. and T.W.-C. edited the manuscript. T.W.-C., M.L.J., S.R.Q., J.E.E., M.P.S., J.L. and C.R.B. supervised the work.
Corresponding author
Ethics declarations
Competing interests
C.R.B. is a co-founder and Scientific Advisory Board member of Palleon Pharmaceuticals, Enable Bioscience, InterVenn Bio and Redwood Bioscience (a subsidiary of Catalent), and a member of the Board of Directors of Eli Lilly and Company. T.W.-C. is a co-founder and scientific advisor of Alkahest Inc. T.W.-C. and A.C.Y. are co-inventors on a patent application related to the work published in this paper. N.O. is affiliated with Calico Life Sciences and has no financial interests to declare.
Additional information
Peer review information Nature thanks Peter Meikle, Christopher Walsh, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Endogenous circulatory proteins detected in the brain parenchyma and characterization of the labelled plasma proteome.
a, Comparison of published perfused brain RNA-seq70 and mass spectrometry-based proteomics71 datasets reveals that 1,446 proteins are present in the hippocampus but are not expressed in the hippocampus. These 1,446 proteins then probably migrate from the periphery into the hippocampus. b, c, The 1,446 proteins present but not expressed in the hippocampus in a are probably derived from the blood, as assessed by tissue-specific expression analysis (TSEA)72 (b) and Gene Ontology Molecular Function (GOMF) and Cell Compartment (GOCC)73,74 (c) (Fisher’s exact test with Benjamini–Hochberg correction). d, Overview of plasma labelling chemistries and detection methods to confirm labelling of hundreds of distinct plasma proteins, limited by detection method. e, Chemoselective labelling of plasma proteins via NHS ester chemistry under non-denaturing conditions (top). Conditions were optimized for each tag to achieve broad and non-perturbative protein labelling. Structures for the small affinity tags biotin and trans-cyclooctene are also shown (bottom). f, Plasma proteins labelled with biotin were incubated on an antibody array and probed with streptavidin. Hundreds of biotinylated proteins were detected across protein groups (n = 6 mice). Specific protein–antibody binding indicates that the labelling did not interfere with protein structure. The signal in the unlabelled plasma array (left) corresponds to biotinylated positive controls to ensure proper antibody printing. g, Plasma proteins labelled with the click moiety trans-cyclooctene were enriched on tetrazine beads before fractionation and mass spectrometry (MS)-based identification. Labelled proteins spanned abundance and sizes, and show no overt bias compared to the overall, unlabelled and detectable plasma proteome (FDR of <0.05, n = 3 mice per group, two-way ANOVA; mean ± s.e.m.).
Extended Data Fig. 2 Enhanced uptake of plasma into the brain.
a, Autoradiography (ARG) and gamma counter quantification of 64Cu-labelled IgG and Alb/IgG-depleted plasma in whole brains (top) and the devascularized hippocampal and cortical parenchyma (bottom) from young (3-month-old) mice 1 h after intravenous ID (7.7 MBq matched dose, about 20 μg) (n = 6 IgG whole, n = 10 plasma whole brain; n = 5 IgG parenchyma, n = 9 plasma parenchyma, two-sided t-test; mean ± s.e.m.). b, 64Cu-labelled IgG and Alb/IgG-depleted plasma detected in the blood of young (3-month-old) and aged (22-month-old) mice at 1, 3 and 20 h after ID (IgG in young n = 6 for all three time points; IgG in aged n = 6 for 1 h and 20 h time points and n = 5 for 3 h time point; plasma in young and plasma in aged n = 10 for 1 h and 20 h time points and n = 5 for 3 h time point; mean ± s.e.m.). c, Time points (1, 3 and 20 h) for gamma counter quantification of 64Cu-labelled IgG and Alb/IgG-depleted plasma in circulating blood from young (3-month-old) and aged (22-month-old) mice after intravenous ID (7.7 MBq matched dose, about 20 μg). t1/2 represents the estimated blood half-life for each condition (n = 5–10). d, Brain regions demarcated by a 3D mouse brain atlas rendering51,52 for in vivo PET signal detection. e, In vivo PET signal detected across brain regions from a in young (3-month-old) mice 20 h after tail-vein intravenous ID of 7.7 MBq (about 20 μg) of 64Cu-labelled IgG or Alb/IgG-depleted plasma. The signal is corrected for activity in a corresponding cardiac blood sample at 20 h (n = 7 young IgG, n = 6 young plasma; *P < 0.05, ***P < 0.001, ****P < 0.0001, two-way ANOVA; mean ± s.e.m.). f, Ex vivo autoradiography assessment of 64Cu-labelled IgG or Alb/IgG-depleted plasma localization in coronal brain sections from tail-vein injected young (-month-old) and aged (22-month-old) mice. Nissl staining (middle) and radioactive signal/Nissl overlay (bottom) to examine anatomical co-registration (the colour bar indicates the radioactive signal from low (black) to high (white) (n = 4 per group).
Extended Data Fig. 3 BBB permeability and homeostasis after plasma injections, and brain uptake of plasma across injection volumes and chemistries.
a, Assessment of perfusion completeness by measuring residual, intravenously injected 2 mDa dextran-FITC (DFITC) in brain tissue. No significant difference between dextran-FITC-injected and PBS-injected young (3-month-old) mice after perfusion (n = 6 PBS unperfused and n = 8 for the others; ****P < 0.0001, one-way ANOVA with Tukey’s multiple comparisons correction; mean ± s.e.m.). b, Circulating 2 mDa dextran-FITC in plasma at the time of death in d (n = 6 PBS unperfused and n = 8 for the others; ****P < 0.0001, one-way ANOVA with Tukey’s multiple comparisons correction; mean ± s.e.m.). c, d, BBB permeability after exposure to 150 μl saline or plasma was probed the next day by injections of 3-kDa (c) and 70-kDa (d) dextran tracers for quantification by fluorescence plate reader. Mild traumatic brain injury (TBI) served as a positive control for BBB leakage (for 3 kDa dextran: n = 8 saline, n = 9 plasma, n = 7 TBI; for 70 kDa dextran: n = 4 saline and TBI, n = 5 plasma; one-way ANOVA with Tukey’s multiple comparisons correction; mean ± s.e.m.). e, Representative images of endogenous immunoglobulin (white) extravasation into the parenchyma after exposure to 150 μl saline or plasma, or TBI75. Scale bars, 50 µm. f, Quantification of endogenous immunoglobulin (IgG) extravasation into the brain parenchyma after exposure to 150 μl saline or plasma, or TBI (n = 4 saline, n = 6 plasma and TBI; ns P = 0.9995, one-way ANOVA with Tukey’s multiple comparisons correction; mean ± s.e.m.). g, Plasma protein concentration of mice injected with 150 μl of plasma, 20 h later at time of death in young (3-month-old) and aged (20–24-month-old) mice used throughout the study compared to plasma concentrations at baseline (n = 35 young baseline, n = 35 young injected, n = 22 aged baseline, n = 32 aged injected; ns (left to right) P = 0.9923, P = 0.0657, P = 0.0657, P = 0.4302, P = 0.5430, two-way ANOVA with Sidak's multiple comparisons test; mean ± s.e.m.). h, t-SNE plot of brain endothelial cells from young (3-month-old) mice at baseline and after exposure to 150 μl of plasma, demonstrating no significant perturbation of the cerebrovascular transcriptome upon plasma transfer (n = 3 mice). i, Representative brain images of Atto 647N-labelled plasma injected at various volumes, assayed 4 h later. 150 μl corresponds to 10–15 mg (0.5 mg/g body weight). CD31 marks brain endothelial cells. Scale bars, 100 µm. j, Representative brain images after Atto 647N-labelled plasma transfusion and stained for mouse albumin (mAlbumin; top) and mIgG (bottom). Minority of capillaries are albumin and IgG+ capillaries (arrowheads). Scale bars, 40 µm (left) and 10 µm (right). k, Atto 647N-labelled plasma (left), Alexa Fluor 647-labelled plasma (middle) and l-azidohomoalanine-labelled plasma, detected by sDIBO-647 click strain-promoted alkyne-azide cycloadditions (SPAAC) slice staining. In vivo labelled l-azidohomoalanine plasma76,77 was extracted and transfused into separate mice under normal (no l-azidohomoalanine) conditions. Scale bars, 20 µm. l, Representative images of after Atto 647N-labelled plasma transfusion and stained for mouse transferrin (top). Scale bars, 50 µm. In addition, quantification of the per cent of plasma+ neurons that also contain transferrin is shown (bottom, n = 4).
Extended Data Fig. 4 Plasma signal in the brain does not arise from free label or degradation product.
a, Representative radio-TLC (thin layer chromatography) of free 64Cu, pre-injection 64Cu-labelled IgG, pre-injection 64Cu-labelled plasma and brain lysates after intravenous injection of 64Cu-labelled plasma. Note the migration of free 64Cu relative to protein. Brain lysates are near the limit of detection, hence the background noise. b, Flow cytometry of fluorescent plasma uptake across all CNS cells. Plasma fluorescence appears only if plasma is appropriately labelled via NHS ester chemistry before intravenous injection (bottom), compared to plasma similarly incubated with fluorophore without a covalent conjugative moiety (carboxy; top). mo, month old. c, Flow cytometry quantification of plasma uptake by CNS cells from young (3-month-old) and aged (22-month-old) mice, as assessed by the per cent (%) of cells that are plasma+ and the MFI of plasma+ cells, 4 h after intravenous injection of 150 μl plasma. Note the lack of signal from carboxy-fluorophore-injected plasma, indicating no detectable confound from non-specifically bound free label (n = 4; two-sided t-test; mean ± s.e.m.). d, Representative images of plasma accumulation in lung tissue and endothelium (CD31+), showing no detectable signal from residual, non-specifically bound free dye in lung tissue lacking a canonical barrier. Scale bars, 20 µm. e, Schematic and representative images of azidohomoalanine (AHA)-incorporated plasma detected in brain vasculature and neurons. Unlike NHS ester labelling, there is no protein-reactive ‘free label’ with AHA-incorporated plasma, as AHA substitutes for methionine at a low rate via the methionyl-tRNA synthetase. AHA-incorporated plasma is dialysed and transfused into recipient mice and brain tissue collected for copper-free click chemistry. Scale bars, 20 µm. f, Fluorescent gels showing minimal protein degradation of an example BBB-permeable ligand, holo-transferrin, 3–4 h after intravenous injection. Human holo-transferrin runs at the expected approximately 75 kDa in size, with its dimer visible in collected plasma samples of injected mice.
Extended Data Fig. 5 Plasma uptake across the healthy brain.
a, Representative sagittal image of plasma suffused in the healthy adult brain. Scale bar, 1,000 µm. b–f, Plasma permeates the vasculature and parenchyma across brain regions, including the hippocampus (b), the cerebral cortex (c), the cerebellum (d), the thalamus (e) and the midbrain (f). Scale bars, 100 µm. g, Representative image of plasma in the median eminence. Scale bar, 10 µm. h, Representative image of plasma (white) uptake in neurons associated with CD31+ vasculature (red) in the hippocampal dentate gyrus. Scale bar, 20 µm. i, Representative volume rendering of plasma distribution in a large artery. Plasma accumulates in focal clusters, characteristic of clathrin-mediated endocytosis78. Scale bar, 50 µm. j, Plasma accumulation in capillary basement membranes and uptake by thin-strand pericytes (arrowheads) 6 h after intravenous injection of 150 μl plasma. Scale bars, 10 µm (representative image of n = 4 young mice). k, Representative image of plasma in the perivascular space, with plasma tracing the outlines of smooth muscle cells. Scale bars, 50 µm (left), 20 µm (right). l, Representative image of plasma suffusing the subarachnoid space, localizing beyond endothelial cells (CD31) but within the glia limitans (AQP4). Scale bar, 10 µm. m, Representative image of a strong plasma signal in the choroid plexus epithelium (Claudin1+), suggesting plasma entry into the CSF and a potential role of the glymphatic system in plasma uptake and clearance. Scale bar, 50 µm. n, 3D reconstruction of plasma (white) uptake specifically in the cytoplasm of neurons, not colocalized with the CD31+ vasculature (red) or GFAP+ astrocytes (green). Scale bars, 15 µm (left), 5 µm (right). o, Plasma uptake by both neuronal soma and processes in the dentate gyrus of the hippocampus, 20 h after intravenous injection of 150 μl plasma. Scale bar, 10 µm (representative image of n = 4 young mice). p, Orthogonal view projections of plasma (white) uptake in the cytoplasm of cortical and hippocampal neurons, beyond the CD31+ vasculature (red). Scale bars, 20 µm (left), 10 µm (right). q, Detection of pSTAT3 (red) in a leptin+ (green) neuron, indicating neuronal binding and response to full-length, intravenously injected leptin. Scale bars, 20 µm.
Extended Data Fig. 6 Contributions to plasma uptake by the BCSFB and the vascular BBB.
a, Lightsheet microscopy of iDISCO-cleared brains to reveal the three-dimensional distribution of plasma (white) uptake across the BCSFB and vascular BBB. b, Co-injection paradigm of plasma into the CSF via either CM or intraventricular injections (ICV), both alongside intravenous (IV) injections. Plasma injected into the CSF (10 µl) and blood (150 µl) were labelled with distinct fluorophores. Injection volumes were based on previous studies41,63,64, representing 25% (CSF) and 7.5% (blood) of total reservoir volumes (for example, 150 µl in 2 ml total circulatory volume). c, Representative coronal image of ICV-injected (green) and IV-injected (white) plasma in the healthy adult brain. ICV-injected plasma is localized near the targeted ventricle. Scale bars, 1,000 µm. d, Representative coronal image of CM-injected (green) and IV-injected (white) plasma in the healthy adult brain. Scale bars, 1,000 µm. e, Representative image of double+ (plasma ICV+ IV+) neurons, where plasma uptake could have arisen via both the BCSFB and the vascular BBB. Scale bars, 40 µm. f, Representative image of single+ (plasma ICV− IV+) neurons, where plasma uptake probably occurred across the vascular BBB. Scale bars, 40 µm. g, Representative image of single+ (plasma CM− IV+) neurons, where plasma uptake probably occurred across the vascular BBB. Scale bars, 40 µm. h, Representative image of cortical vasculature with punctated vesicles (arrowheads) arising only from IV-injected plasma (red). The diffuse signal in subarachnoid and perivascular spaces arise from IV-injected plasma (green). Scale bars, 40 µm. i, Representative image of an acutely generated ex vivo brain slice demonstrating intracellular lysosomal uptake (pHrodo555) of a subset of total IV-injected plasma (Atto647) in the brain vasculature. Scale bars, 40 µm. j, 3D reconstruction of plasma (white) within CD31+ (red) vasculature. Plasma puncta are under the endothelial CD31+ cell surface (left) but in front of intracellular nuclei (right), indicating cytoplasmic localization of plasma proteins within the brain vasculature. Scale bars, 3 µm. k, 3D reconstruction of plasma (white) within CD31+ (red) vasculature, as in j. Plasma puncta are under the luminal endothelial CD31+ cell surface (left) but not visible from the abluminal side (right), indicating cytoplasmic localization of plasma proteins within the brain vasculature. Scale bars, 5 µm. l, Gene expression of putative RMT receptors, clathrin components, and caveolar components and their inhibitor in choroid plexus and lymphatic endothelial cells of young (2–3 mo) and aged (20–24 mo) mice79,80. Relative Z-scored values are indicated in graded yellow (high) or blue (low).
Extended Data Fig. 7 Purity and consistency of sorted BECs for scRNA-seq and correlative plasma uptake analysis.
a, Sorted BECs from young (3-month-old) mice were further filtered to purity using scRNA-seq analysis using canonical cell-type markers (n = 3 young mice). b, Violin plot of the number of genes expressed per BEC within each biological replicate. Cells in red are outliers (745 total BECs from n = 3 young mice). Violin box plots show the median and the 25th to 75th percentiles, and the whiskers indicate the minimum and maximum. c, t-SNE plot with BECs from separate mice coloured distinctly, showing consistency in transcriptomes across replicates (745 total BECs from n = 3 young mice). d, Violin plot of the number of genes expressed per BEC by zonation. Cells in red are outliers (745 total BECs from n = 3 young mice). Violin box plots show the median and the 25th to 75th percentiles, and the whiskers indicate the minimum and maximum. e, t-SNE plot demonstrating BEC separation by arterial, capillary and venous zonation (n = 3 young mice). f, Pathway enrichment analysis of ‘Correlate’ and ‘Anticorrelate’ genes, with the number of genes in each pathway listed (from n = 3 young mice, GeneAnalytics analysis)81. ECM, extracellular matrix; SLC, solute carrier. g, Gene Ontology cellular component enrichment analysis of ‘Correlate’ and ‘Anticorrelate’ genes (from n = 3 young mice, GOrilla exact mHG P-value computation)82. h, Robust PCA plots of example ‘Correlate’ and ‘Anticorrelate’ gene expression across BECs. Each dot is an individual cell, and gene expression levels are indicated by the colour spectrum (log10 CPM, 745 total BECs from n = 3 young mice). i, Tight junction gene expression from RNA-seq of BECs from young (3 mo) and aged (20 mo) mice (n = 6). Relative Z-scored values are indicated in graded yellow (high) or blue (low). j, SLC transporter gene expression from RNA-seq of BECs from young (3 mo) and aged (20 mo) mice (n = 6). Relative Z-scored values are indicated in graded yellow (high) or blue (low). The SLC genes shown are derived from two comprehensive reviews83,84.
Extended Data Fig. 8 Lipidomics companion to the decrease in MFSD2A expression in the aged brain vasculature.
a, PC analysis plot of brain microvessel lipidomes from young (3 mo) and aged (20 mo) mice. There were 25 lipids significantly upregulated and 33 lipids downregulated with age (n = 4, each n is a pool of 4 mice (16 young and 16 aged total), FDR-corrected q < 0.05, Permutation-based FDR of two-sided t-test). b, Absolute abundance (concentrations) of measured microvessel lipid classes with age (n = 4, each n is a pool of 4 mice, Benjamini, Krieger and Yekutieli FDR-corrected two-sided t-test; mean ± s.e.m.). DG is not listed as only one DG species was detected. c, Mole % of MFSD2A-regulated phospholipids19 (PC, PE, PS and LPE) across and within phospholipid classes. Concentrations of individual lipid species were derived with spike-in standards and presented as a quantitative mole % of DHA fatty acid (FA) of all fatty acids in the given class68 or across PC, PE, PS and LPE classes (right, ‘Combined’) (n = 4, each n is a pool of 4 mice, two-sided t-test; mean ± s.e.m.). d, Hierarchical clustering of lipids (by concentration) of microvessels from young (3 mo) and aged (20 mo) mice (n = 4; each sample is a pool of 4 mice) normalized by Z-score. All lipids differentially abundant with an FDR-corrected q < 0.1 are shown (permutation-based FDR of two-sided t-test), and MFSD2A-regulated DHA-phospholipids are labelled.
Extended Data Fig. 9 Specificity and effect of pericyte loss in the aged brain vasculature and working model of BBB transcytosis with age.
a, Representative images of AQP4+ vascular astrocytes (green) and CD31+ endothelial cells in the cerebral cortex of young (3 mo) and aged (23 mo) mice. Scale bars, 40 µm. b, Quantification of vascular astrocyte coverage in the cerebral cortex vasculature of young (3 mo) and aged (23 mo) mice (n = 5 young and n = 7 aged; two-sided t-test; mean ± s.e.m.). c, Quantification of endothelial cell density in the cerebral cortex vasculature of young (3 mo) and aged (23 mo) mice (n = 5 young and n = 7 aged; two-sided t-test; mean ± s.e.m.). d, CD31 gene expression from RNA-seq of BECs from young (3 mo) and aged (20 mo) mice (ns P = 0.699, two-sided t-test; mean ± s.e.m.). e, Gene expression of brain pericyte and astrocyte markers from young (2–3 mo) and aged (21–22 mo) mice85 (ns (exact P-values can be found in Supplementary Table 6 of ref. 85), n = 8 young and aged, FDR-corrected MAST test). f, Correlation of changes in gene expression between BECs from aged (20 mo) and young (3 mo) and BECs from pericyte knockout Pdgfbret/ret mice20 and age-matched wild-type mice (R = 0.35, P < 10−15; the blue line is the simple linear regression and the fill is the 95% confidence interval, Spearman’s correlation; n = 4 Pdgfbret/ret mice and n = 3 controls, and n = 6 young and aged mice). g, Representative images of calcified nodules (Alizarin Red staining) detected in aged (23 mo) but not young (3 mo) mice, recapitulating nodules seen in pericyte-deficient Pdgfbret/ret mice (representative image of n = 21 mice per age). Scale bars, 330 µm. h, Representative images of vascular calcifications in the aged (23 mo) brain. Risedronate (white) accumulation in lectin+ (red) vasculature by immunofluorescence staining. i, Representative images of type I collagen expression (green) in lectin+ vasculature (red) in the cerebral cortex of young (3 mo) and aged (23 mo) mice. Scale bars, 40 µm (left), 20 µm (right). j, Dot plot representation of cell-surface (from Uniprot), druggable gene candidates on BECs. Genes are plotted by their degree of BBB specificity15 and correlation with plasma uptake. Genes are coloured by their upregulation (brown) or downregulation (green) with age. k, Working model of BBB transcytosis with age. In healthy adults, BECs express higher levels of receptors and components of clathrin-coated pits86,87 to transport select circulatory proteins via RMT. With age, pericytes degenerate, promoting vascular calcification and a shift in endothelial transport from ligand-specific RMT to caveolar transcytosis. Caveolar transcytosis is non-specific87, rendering the aged BBB ‘leaky’ to neurotoxic proteins excluded in health, such as fibrin(ogen), thrombin and autoantibodies25,27.
Extended Data Fig. 10 Rationale for ALPL inhibitor treatment in aged brains and purity of sorted aged BECs for scRNA-seq after pharmacological ALPL inhibition.
a, t-SNE and violin plot showing scRNA-seq analysis of Alpl expression in CNS cells, log-normalized counts per million reads (CPM). Alpl is expressed mainly in BECs (n = 7 young mice). Data from the Tabula Muris Consortium54. OPC, oligodendrocyte precursor cell. b, Alpl expression across endothelial cells (ECs) and non-ECs in the CNS, periphery and tissue culture (transcripts per million (TPM)). Alpl is specific to the brain endothelial and expression is lost upon culture. Data from the Vascular Endothelial Cell Trans-omics Resource Database (https://markfsabbagh.shinyapps.io/vectrdb/)88. c, Correlation between Alpl expression in young BECs and plasma uptake, from combined flow cytometry index sorting and scRNA-seq. The blue line denotes linear regression and the fill denotes the confidence interval, Spearman’s correlation. Non-Alpl-expressing BECs were excluded (n = 3 young (3 mo) mice). d, Alpl expression increases specifically in capillaries with age (n = 6 mice, FDR-corrected Mann–Whitney U-test). Analysis based on published data57. e, ALPL protein expression in young (3 mo) and aged (23 mo) brains. Scale bars, 1,000 µm. f, Sorted BECs from aged (22 mo) mice treated with an ALPL inhibitor or vehicle were further filtered to purity using scRNA-seq analysis using canonical cell-type markers (n = 4 mice per group). g, BECs were assigned to arterial, capillary and venous vessel segments based on vasculature zonation markers (n = 4 mice per group)16,57. h, Gene Ontologies (GO) enriched in differentially expressed genes in capillaries upon ALPL inhibitor versus vehicle treatment (Enrichr analysis; the number of genes in the pathway are shown in brackets)82.
Extended Data Fig. 11 Validation of ALPL inhibitor treatment and relevance to the human vasculature and Alzheimer’s disease.
a, b, Flow cytometry quantification of BEC uptake of human holo-transferrin (hu-Tf) from aged (a) or TfR Ab from aged (b) mice treated with vehicle (grey) or ALPL inhibitor (green) via IP injections twice daily for 3 days, as assessed by the percent of BECs that are tracer+ and the relative MFI of tracer+ BECs (for hu-Tf: n = 6 mice vehicle, n = 5 ALPL inhibitor; for TfR Ab: n = 8; two-sided t-test; mean ± s.e.m.). Tracer refers to hu-Tf or TfR Ab. c, Representative images of human holo-Tf+ neurons detected in the cortex of mice treated with ALPL inhibitor. Scale bar, 40 µm. d, e, Circulating human holo-Tf (d) and TfR Ab (e) in blood plasma upon time of death to control for and ensure no differences in the injected amount (for hu-Tf+: n = 6 mice vehicle, n = 5 ALPL inhibitor; for TfR Ab: n = 9; two-sided t-test; mean ± s.e.m.). f, As in a and b, but for the 3-kDa dextran tracer that probes for disruptions in paracellular permeability (n = 6 mice vehicle, n = 5 ALPL inhibitor; two-sided t-test; mean ± s.e.m.). g, Overall cerebrovascular morphology, as assayed by CD31 staining, from aged mice (22 mo) treated with vehicle or an ALPL inhibitor. Scale bars, 1,000 µm. h, i, As in a and b, but for plasma uptake in BECs from aged mice (22 mo, n = 6) (h) and parenchymal cells from young mice (3 mo, n = 4; two-sided t-test; mean ± s.e.m.) (i). j, Alpl gene expression in various cell types of the human CNS, showing exclusive expression in endothelial cells. Data from Barres lab RNA-seq (http://www.brainrnaseq.org; mean ± s.e.m.)89. k, ALPL protein expression localized to the human brain vasculature in The Human Protein Atlas (http://www.proteinatlas.org)90,91. l, The Alpl single-nucleotide polymorphism (SNP) rs1767429 shows association with Alzheimer’s disease (over 160,000 individuals, genome-wide association study (GWAS) analysis)92.
Supplementary information
Supplementary Information
This file contains Supplemental Figure 1 and Supplemental Table 1 with additional References. Supplemental Figure shows the flow cytometry gating strategy to analyze plasma uptake across CNS cell types. a, b, c, d. Representative gating for plasma+ (a), NeuN+ neurons; (b), Thy1 (CD90)+ neurons; (c), ACS2-A+ astrocytes; and (d), CD31+/ CD45- brain endothelial cells (BECs). e, Representative gating for CD31+/ CD45- brain endothelial cells and CD31-/ CD45- parenchymal cells from aged mice (22 m.o.) after in vivo transfer of plasma, human holo-transferrin, or transferrin receptor antibody with or without ALPL inhibitor treatment. Plasma, human holo-transferrin, and transferrin receptor antibody (“tracer”) gates were set off FMO negative controls (uninjected) to avoid confounding autofluorescence. Examples shown are from mice injected with transferrin receptor antibody. Supplementary Table 1 shows the tracers injected or perfused to probe BBB permeability. A review of tracers used to study blood-brain barrier permeability in health and disease with specific findings, animal models/ study cohorts, disease, and detection methods.
Supplementary Table 2
scRNAseq meta-data Single-cell RNA sequencing meta-data of brain endothelial cells isolated from young (3 m.o.) mice by flow cytometry.
Supplementary Table 3
Plasma-gene correlations Single-cell RNA sequencing gene-plasma correlations from brain endothelial cells isolated from young (3 m.o.) mice by flow cytometry. Spearman correlations between 19,899 genes sequenced and level of plasma uptake across 745 brain endothelial cells.
Supplementary Table 4
Microvessel lipidomics Concentrations of individual lipid species from LC-MS lipidomic profiling of brain microvessels isolated from young (3 m.o.) and aged (20 m.o.) mice.
Supplementary Table 5
scRNAseq ALPL inhibition Droplet-based single-cell RNA sequencing of sorted brain endothelial cells from aged (22 m.o.) mice treated with ALPL inhibitor or vehicle control.
Supplementary Video 1
iDISCO Plasma Whole Brain Lighsheet microscopy of iDISCO-cleared brains to reveal the three-dimensional distribution of plasma (white) uptake across the blood-CSF and blood-brain barriers.
Source data
Rights and permissions
About this article
Cite this article
Yang, A.C., Stevens, M.Y., Chen, M.B. et al. Physiological blood–brain transport is impaired with age by a shift in transcytosis. Nature 583, 425–430 (2020). https://doi.org/10.1038/s41586-020-2453-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2453-z
This article is cited by
-
Blood–brain borders: a proposal to address limitations of historical blood–brain barrier terminology
Fluids and Barriers of the CNS (2024)
-
Proteomics of mouse brain endothelium uncovers dysregulation of vesicular transport pathways during aging
Nature Aging (2024)
-
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
Nature Reviews Gastroenterology & Hepatology (2024)
-
Circulating small extracellular vesicles mediate vascular hyperpermeability in diabetes
Diabetologia (2024)
-
Healthy blood, healthy brain: a window into understanding and treating neurodegenerative diseases
Journal of Neurology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.