Abstract
Light-driven sodium pumps actively transport small cations across cellular membranes1. These pumps are used by microorganisms to convert light into membrane potential and have become useful optogenetic tools with applications in neuroscience. Although the resting state structures of the prototypical sodium pump Krokinobacter eikastus rhodopsin 2 (KR2) have been solved2,3, it is unclear how structural alterations over time allow sodium to be translocated against a concentration gradient. Here, using the Swiss X-ray Free Electron Laser4, we have collected serial crystallographic data at ten pump–probe delays from femtoseconds to milliseconds. High-resolution structural snapshots throughout the KR2 photocycle show how retinal isomerization is completed on the femtosecond timescale and changes the local structure of the binding pocket in the early nanoseconds. Subsequent rearrangements and deprotonation of the retinal Schiff base open an electrostatic gate in microseconds. Structural and spectroscopic data, in combination with quantum chemical calculations, indicate that a sodium ion binds transiently close to the retinal within one millisecond. In the last structural intermediate, at 20 milliseconds after activation, we identified a potential second sodium-binding site close to the extracellular exit. These results provide direct molecular insight into the dynamics of active cation transport across biological membranes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Resting state coordinates and structure factors have been deposited in the PDB database under accession code 6TK7 (acidic pH) and 6TK6 (neutral pH). Together with the neutral pH resting state structure, the light-activated data for all time points (800 fs, 2 ps, 100 ps, 1 ns, 16 ns, 1μs, 30 μs, 150 μs, 1ms and 20 ms) were deposited in the mmCIF file. For the refined structures using combined data (800 fs + 2 ps, 1 ns + 16 ns and 30 μs + 150 μs) and single (1 ms and 20 ms) light-activated data sets, coordinates, light amplitudes, dark amplitudes and extrapolated structure factors have been deposited in the PDB database under accession codes 6TK5 (800 fs + 2 ps), 6TK4 (1 ns + 16 ns), 6TK3 (30 μs + 150 μs), 6TK2 (1 ms) and 6TK1 (20 ms).
References
Inoue, K. et al. A light-driven sodium ion pump in marine bacteria. Nat. Commun. 4, 1678 (2013).
Kato, H. E. et al. Structural basis for Na+ transport mechanism by a light-driven Na+ pump. Nature 521, 48–53 (2015).
Gushchin, I. et al. Crystal structure of a light-driven sodium pump. Nat. Struct. Mol. Biol. 22, 390–395 (2015).
Milne, C. et al. SwissFEL: The Swiss X-ray Free Electron Laser. Appl. Sci. (Basel) 7, 720 (2017).
Poulsen, S. B., Fenton, R. A. & Rieg, T. Sodium–glucose cotransport. Curr. Opin. Nephrol. Hypertens. 24, 463–469 (2015).
Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213–1225 (2015).
Govorunova, E. G., Sineshchekov, O. A., Li, H. & Spudich, J. L. Microbial rhodopsins: diversity, mechanisms, and optogenetic applications. Annu. Rev. Biochem. 86, 845–872 (2017).
Terakita, A. The opsins. Genome Biol. 6, 213 (2005).
Grimm, C., Silapetere, A., Vogt, A., Bernal Sierra, Y. A. & Hegemann, P. Electrical properties, substrate specificity and optogenetic potential of the engineered light-driven sodium pump eKR2. Sci. Rep. 8, 9316 (2018).
Hoque, M. R. et al. A chimera Na+-pump rhodopsin as an effective optogenetic silencer. PLoS One 11, e0166820 (2016).
Konno, M. et al. Mutant of a light-driven sodium ion pump can transport cesium ions. J. Phys. Chem. Lett. 7, 51–55 (2016).
Inoue, K. et al. Red-shifting mutation of light-driven sodium-pump rhodopsin. Nat. Commun. 10, 1993 (2019).
Inoue, K., Konno, M., Abe-Yoshizumi, R. & Kandori, H. The role of the NDQ motif in sodium-pumping rhodopsins. Angew. Chem. Int. Edn Engl. 54, 11536–11539 (2015).
Tahara, S. et al. Ultrafast photoreaction dynamics of a light-driven sodium-ion-pumping retinal protein from Krokinobacter eikastus revealed by femtosecond time-resolved absorption spectroscopy. J. Phys. Chem. Lett. 6, 4481–4486 (2015).
Tahara, S. et al. Origin of the reactive and nonreactive excited states in the primary reaction of rhodopsins: pH dependence of femtosecond absorption of light-driven sodium ion pump rhodopsin KR2. J. Phys. Chem. B 122, 4784–4792 (2018).
Hontani, Y. et al. The photochemistry of sodium ion pump rhodopsin observed by watermarked femto- to submillisecond stimulated Raman spectroscopy. Phys. Chem. Chem. Phys. 18, 24729–24736 (2016).
Kaur, J. et al. Solid-state NMR analysis of the sodium pump Krokinobacter rhodopsin 2 and its H30A mutant. J. Struct. Biol. 206, 55–65 (2019).
Chen, H.-F. et al. Time-resolved FTIR study of light-driven sodium pump rhodopsins. Phys. Chem. Chem. Phys. 20, 17694–17704 (2018).
Asido, M. et al. Time-resolved IR spectroscopy reveals mechanistic details of ion transport in the sodium pump Krokinobacter eikastus rhodopsin 2. Phys. Chem. Chem. Phys. 21, 4461–4471 (2019).
Kovalev, K. et al. Structure and mechanisms of sodium-pumping KR2 rhodopsin. Sci. Adv. 5, eaav2671 (2019).
Barends, T. R. M. et al. Direct observation of ultrafast collective motions in CO myoglobin upon ligand dissociation. Science 350, 445–450 (2015).
Tenboer, J. et al. Time-resolved serial crystallography captures high-resolution intermediates of photoactive yellow protein. Science 346, 1242–1246 (2014).
Pande, K. et al. Femtosecond structural dynamics drives the trans/cis isomerization in photoactive yellow protein. Science 352, 725–729 (2016).
Nango, E. et al. A three-dimensional movie of structural changes in bacteriorhodopsin. Science 354, 1552–1557 (2016).
Nogly, P. et al. Retinal isomerization in bacteriorhodopsin captured by a femtosecond X-ray laser. Science 361, eaat0094 (2018).
Weinert, T. et al. Proton uptake mechanism in bacteriorhodopsin captured by serial synchrotron crystallography. Science 365, 61–65 (2019).
Nass Kovacs, G. et al. Three-dimensional view of ultrafast dynamics in photoexcited bacteriorhodopsin. Nat. Commun. 10, 3177 (2019).
Kato, Y., Inoue, K. & Kandori, H. Kinetic analysis of H+–Na+ selectivity in a light-driven Na+-pumping rhodopsin. J. Phys. Chem. Lett. 6, 5111–5115 (2015).
Shannon, R. D. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Kandori, H., Inoue, K. & Tsunoda, S. P. Light-driven sodium-pumping rhodopsin: a new concept of active transport. Chem. Rev. 118, 10646–10658 (2018).
Suomivuori, C.-M., Gamiz-Hernandez, A. P., Sundholm, D. & Kaila, V. R. I. Energetics and dynamics of a light-driven sodium-pumping rhodopsin. Proc. Natl Acad. Sci. USA 114, 7043–7048 (2017).
Nishimura, N., Mizuno, M., Kandori, H. & Mizutani, Y. Distortion and a strong hydrogen bond in the retinal chromophore enable sodium-ion transport by the sodium-ion pump KR2. J. Phys. Chem. B 123, 3430–3440 (2019).
Ourmazd, A. Cryo-EM, XFELs and the structure conundrum in structural biology. Nat. Methods 16, 941–944 (2019).
Vogt, A. et al. Engineered passive potassium conductance in the KR2 sodium pump. Biophys. J. 116, 1941–1951 (2019).
Abe-Yoshizumi, R., Inoue, K., Kato, H. E., Nureki, O. & Kandori, H. Role of Asn112 in a light-driven sodium ion-pumping rhodopsin. Biochemistry 55, 5790–5797 (2016).
Gushchin, I. et al. Structure of the light-driven sodium pump KR2 and its implications for optogenetics. FEBS J. 283, 1232–1238 (2016).
Zheng, H. et al. CheckMyMetal: a macromolecular metal-binding validation tool. Acta Crystallogr. D 73, 223–233 (2017).
Zheng, H., Chruszcz, M., Lasota, P., Lebioda, L. & Minor, W. Data mining of metal ion environments present in protein structures. J. Inorg. Biochem. 102, 1765–1776 (2008).
Chovancova, E. et al. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLOS Comput. Biol. 8, e1002708 (2012).
James, D. et al. Improving high viscosity extrusion of microcrystals for time-resolved serial femtosecond crystallography at X-ray lasers. J. Vis. Exp. 144, e59087 (2019).
Weierstall, U. et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat. Commun. 5, 3309 (2014).
Claesson, E. et al. The primary structural photoresponse of phytochrome proteins captured by a femtosecond X-ray laser. eLife 9, e53514 (2020).
White, T. A. et al. CrystFEL: a software suite for snapshot serial crystallography. J. Appl. Crystallogr. 45, 335–341 (2012).
White, T. A. et al. Recent developments in CrystFEL. J. Appl. Crystallogr. 49, 680–689 (2016).
Gevorkov, Y. et al. XGANDALF - extended gradient descent algorithm for lattice finding. Acta Crystallogr. A 75, 694–704 (2019).
Ginn, H. M. et al. TakeTwo: an indexing algorithm suited to still images with known crystal parameters. Acta Crystallogr. D 72, 956–965 (2016).
Tickle, I. J. et al. Staraniso. http://staraniso.globalphasing.org/cgi-bin/staraniso.cgi (2020).
Berman, H., Henrick, K. & Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Biol. 10, 980 (2003).
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Heberle, J., Büldt, G., Koglin, E., Rosenbusch, J. P. & Landau, E. M. Assessing the functionality of a membrane protein in a three-dimensional crystal. J. Mol. Biol. 281, 587–592 (1998).
Lórenz-Fonfría, V. A. et al. Transient protonation changes in channelrhodopsin-2 and their relevance to channel gating. Proc. Natl Acad. Sci. USA 110, E1273–E1281 (2013).
Ehrenberg, D. et al. The two-photon reversible reaction of the bistable jumping spider rhodopsin-1. Biophys. J. 116, 1248–1258 (2019).
Schultz, B.-J., Mohrmann, H., Lórenz-Fonfría, V. A. & Heberle, J. Protein dynamics observed by tunable mid-IR quantum cascade lasers across the time range from 10 ns to 1 s. Spectrochim. Acta A 188, 666–674 (2018).
Senn, H. M. & Thiel, W. QM/MM methods for biomolecular systems. Angew. Chem. Int. Edn Engl. 48, 1198–1229 (2009).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Kossmann, S. & Neese, F. Efficient structure optimization with second-order many-body perturbation theory: the RIJCOSX-MP2 method. J. Chem. Theory Comput. 6, 2325–2338 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Grimme, S. A simplified Tamm–Dancoff density functional approach for the electronic excitation spectra of very large molecules. J. Chem. Phys. 138, 244104 (2013).
Metz, S., Kästner, J., Sokol, A., Keal, T. & Sherwood, P. ChemShell—a modular software package for QM/MM simulations. WIREs Comput. Mol. Sci. 4, 101–110 (2014).
Balashov, S. P. et al. Light-driven Na+ pump from Gillisia limnaea: a high-affinity Na+ binding site is formed transiently in the photocycle. Biochemistry 53, 7549–7561 (2014).
Noguchi, T., Ono, T.-A. & Inoue, Y. Direct detection of a carboxylate bridge between Mn and Ca2+ in the photosynthetic oxygen-evolving center by means of Fourier transform infrared spectroscopy. Biochim. Biophys. Acta 1228, 189–200 (1995).
Nara, M. et al. Infrared studies of interaction between metal ions and Ca2+-binding proteins. Marker bands for identifying the types of coordination of the side-chain COO− groups to metal ions in pike parvalbumin (pI = 4.10). FEBS Lett. 349, 84–88 (1994).
Nara, M., Morii, H. & Tanokura, M. Coordination to divalent cations by calcium-binding proteins studied by FTIR spectroscopy. Biochim. Biophys. Acta 1828, 2319–2327 (2013).
Furutani, Y., Murata, T. & Kandori, H. Sodium or lithium ion-binding-induced structural changes in the K-ring of V-ATPase from Enterococcus hirae revealed by ATR-FTIR spectroscopy. J. Am. Chem. Soc. 133, 2860–2863 (2011).
Wickstrand, C. et al. A tool for visualizing protein motions in time-resolved crystallography. Structural Dynamics (Melville, N.Y.) 7, 024701 (2020).
Kim, K. et al. Crystal structure and functional characterization of a light-driven chloride pump having an NTQ motif. Nat. Commun. 7, 12677 (2016).
Kolbe, M., Besir, H., Essen, L. O. & Oesterhelt, D. Structure of the light-driven chloride pump halorhodopsin at 1.8 Å resolution. Science 288, 1390–1396 (2000).
Wickstrand, C. et al. Bacteriorhodopsin: structural insights revealed using X-ray lasers and synchrotron radiation. Annu. Rev. Biochem. 88, 59–83 (2019).
Gerwert, K., Freier, E. & Wolf, S. The role of protein-bound water molecules in microbial rhodopsins. Biochim. Biophys. Acta 1837, 606–613 (2014).
Acknowledgements
This project was funded by the following agencies: The Swiss National Science Foundation project grants 31003A_141235 and 31003A_159558 (to J.S.), PZ00P3_174169 (to P.N.), 310030_192780 (to X.D.) and 310030B_173335 (to G.F.X.S.). We further acknowledge support by the NCCR:MUST (to C.M. and J.S.). The German Research Foundation supported the work through SFB-1078, project B3 and SPP-1926, HE 2063/6-1 (to J.H). This project has received funding from the European Union’s Horizon 2020 research and innovation program under Marie-Sklodowska-Curie grant agreements 701646 and 701647. I.S. acknowledges funding by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant no. 678169 ‘PhotoMutant’). I.S. thanks the SFB 1078 ‘Protonation Dynamics in Protein Function’ for the Mercator fellowship. R.K.K. acknowledges support from the Lady Davis Trust for the Arskin postdoctoral fellowship. We thank N. Varma for discussions on TR-SFX sample jetting and the Macromolecular Crystallography group for support during testing of crystals at the Swiss Light Source. We further thank everybody involved in ensuring the smooth operation of the Swiss X-ray Free Electron Laser during our experiments.
Author information
Authors and Affiliations
Contributions
The project was initiated and coordinated by J.S. following input on crystallographic data evaluation from T.W., quantum chemical calculations from I.S., pump–probe experiments at the Alvra endstation from C.M. and time-resolved spectroscopy from J.H. G.F.X.S. coordinated and supported crystallographic applications at SwissFEL and contributed to discussions throughout the project. The KR2 expression construct was cloned by R.M.B. The protein was expressed, prepared and crystallized by P.S. with help from A.F. A constant supply of sample during the beamtime was secured with the help of A.F., T.G., M.W., D.E., D.K. and R.M.B. using protocols developed by P.S. Crystal injection was optimized by P.S. and D.J. The lipidic cubic phase injector was operated and aligned during the beamtime by D.J., F.D., I.M. and D.G. The endstation including the laser system was aligned and operated by P.S., K.N., G.K., C.C. and C.M., who also designed the Alvra prime pump–probe station. C.A. and P.J.M.J. operated the timing diagnostics during the experiment. The SFX data analysis pipeline was built and operated by D.O. and K.N. Data processing during the beamtime was done by T.W., P.N. and S.B. S.M., V.P. and X.D. recorded progress during data collection and/or in a previous test beamtime. Final structures were refined by P.S. and T.W. and interpreted together with J.S. Quantum chemical calculations were done by R.K.K. and I.S. The time-resolved spectroscopic experiments were done by D.E. and P.S. and interpreted together with J.H.. The manuscript was written by J.S. with direct contributions from P.S., T.W., D.E., J.H., R.K.K. and I.S. and with further suggestions from most of the other authors. All authors read and acknowledged the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 TR-SFX sample preparation scheme and characterization.
a, Two lipidic phases, the first containing KR2 crystals from which the acidic buffer had been washed away and the second containing soaking buffer, were mixed through a three-way coupler40. The sodium concentration was adjusted to 150 mM and tests with litmus paper indicate a pH close to 8 in the final mixture. b, Left, crystals after TR-SFX sample preparation with varying Tris buffers with pH values from 7.0 to 9.0. Right, KR2 in 200 mM Tris, 150 mM NaCl, 0.02% DDM, 0.004% CHS solution with varied pH of Tris buffer. Note that crystals grown in LCP reach a red colour after mixing with Tris pH 9.0, whereas the solution reaches red colour at pH 8.0. This shift is probably due to residual buffering capacity from the original crystallization conditions, as confirmed by a litmus paper test. c, Size distribution of crystals determined by microscopy inspection after TR-SFX sample preparation. d, Microscopy picture of KR2 crystals grown at acidic pH and in the absence of NaCl; after washing out the crystallization buffer; the TR-SFX sample prepared via the procedure shown in a; and LCP with KR2 crystals soaked directly as control. The colour change upon increasing pH was confirmed in five independent experiments. e, Overview of TR-IR traces and visible absorption spectra obtained from blue KR2 crystals in the original acidic crystallization condition, red crystals prepared in analogy to the TR-SFX experiment, and in hydrated film at pH 8 in the presence of sodium chloride (solution). The corresponding lower panels show a kinetic analysis of KR2 intermediates obtained by global fit analysis of the spectroscopic data. Time constants are given in parentheses. Under acidic conditions, KR2 in crystals exhibits an accelerated photocycle. In treated crystals, the critical deprotonation step in the M intermediate occurs with similar kinetics as in purified KR2. f, g, Detailed view of the retinal binding pocket in the serial crystallographic room temperature structures of KR2 obtained from blue crystals at acidic pH (f) and red crystals after soaking in neutral pH and NaCl (g). Critical hydrogen bonds with a distance of ≤3.2 Å are indicated by black dotted lines. Arrows signify the distance from the SB to w406 and the D116 counterion. In neutral conditions, w406 has shifted away from the SB while D116 and N112 are now within hydrogen bonding distance. The resulting change in electrostatic environment is responsible for the colour change, as reported previously2.
Extended Data Fig. 2 Spectroscopic analysis of sodium binding mode.
a, b, Time-resolved IR absorption changes from KR2 microcrystals at pH 8 (a) and at pH 4 (b) recorded with tunable quantum cascade lasers as described54. The maximum concentration of the O intermediate is reached at around 1–20 ms after pulsed excitation at pH 8 and is characterized by specific marker bands that are absent at pH 4, in particular the C=C stretching vibrational band of retinal at 1,516 cm−1 of the O state. The band at 1,688 cm−1 has previously been suggested to originate from the C=O stretching mode as a result of sodium binding to an asparagine residue, presumably N11261. The band at 1,554 cm−1 is tentatively assigned to the asymmetric stretching vibration of a carboxylate that rises upon binding of a sodium ion in bidendate or pseudo-bridging fashion where one of the carboxylate oxygens is interacting with another partner62,63. For a detailed analysis of the ligation, the corresponding symmetrical mode needs to be assigned, as the frequency difference between the COO− asymmetric and symmetric stretch is dependent on the mode of sodium ligation64,65. c, O(like)-KR2 (ground state) difference spectra recorded under different conditions. Spectra have been scaled to the ground-state bleaching band measured between 1,530 and 1,540 cm−1. It is well-established that KR2 operates exclusively as a sodium pump at neutral pH and in the presence of sodium ions. KR2 acts as proton pump in the presence of KCl but has no (known) function at pH 41,28. It is evident that the band at 1,688 cm−1 is most pronounced if sodium is pumped, which supports the assignment to the C=O stretching vibration of N112 upon binding of sodium. d, Time traces of the ethylenic stretch of the O state vibrating at 1,518 cm–1 and the two candidate vibrational bands at 1,420 cm–1 and 1,392 cm−1 of the symmetric carboxylate stretching vibration. The band at 1,420 cm–1 exhibits similar kinetic behaviour to the one at 1,518 cm–1. Hence, the former vibrational band is tentatively assigned as the symmetrical mode that relates to the asymmetrical vibration at 1,554 cm−1 of deprotonated D251 upon ligation of a sodium ion. The difference in frequency between the symmetric and asymmetric modes is with 135 cm−1 at the edge of binding in bidentate to pseudobridging mode of model compounds62,64,65. This indicates that the two oxygens of D251 are not equidistant from the sodium ion. Such asymmetric ligation is expected in the heterogenous environment of the protein interior as documented by our X-ray structures in the millisecond regime.
Extended Data Fig. 3 Comparison of data truncation schemes and changes in retinal over time.
a, Top, Fo − Fc simulated annealing omit maps of the resting state at 4σ. No truncation (left) shows the map using all data up to 1.6 Å resolution along a*, b* and c*. Spherical truncation (middle) shows the map using all data up to 2.2 Å resolution along a*, b* and c*. Anisotropic truncation (right) shows the map using data up to 2.3 Å, 2.2 Å and 1.6 Å resolution along a*, b* and c*, respectively, as truncated by the staraniso server47. Bottom, Fo(light) − Fo(dark) difference density maps of the 1-ms time delay at the region around retinal and V117 at 3σ. The structure is shown as sticks (salmon, resting state; cyan, 1 ms refined structure). No truncation (left) shows the map using all data up to 1.6 Å resolution. Spherical truncation (middle) shows the map using all data up to 2.2 Å resolution. Anisotropic truncation (right) shows the map using data up to 1.6 Å resolution in c* as truncated by the staraniso server. Overall, the truncated data result in better electron density maps (both for 2Fo − Fc maps and Fo(light) − Fo(dark) difference maps), with finer features being resolved. This effect is probably because noise along the missing directions is removed when compared to no truncation, while retaining the high-resolution data along c* when compared to spherical truncation. b, The evolution of electron density around the retinal chromophore over time. Retinal and K255 of the refined structures are shown as sticks and the electron density is displayed around them (blue mesh, 2σ). Top, original 2Fo − Fc electron density map; panels below show extrapolated 2Fextra − Fc maps. The extrapolated maps allow us to follow retinal isomerization in detail. In the dark state (top), the middle section or the retinal polyene chain is slightly bent downwards. In the picoseconds range, the isomerization is completed and the polyene appears to be straightened. In our ultrafast data, we did not observe retinal with a pronounced twist in the C13=C14 bond as in bR, with retinal in KR2 reaching a near planar 13-cis conformation much earlier along the activation pathway. In the time delays from nanoseconds to milliseconds, the electron density reveals a bend in the retinal molecule resulting from two planes that are twisted against each other. While the exact dihedral angles cannot be refined realistically on the basis of the extrapolated data, the bend seems to originate from the C9=C10–C11=C12 dihedral angle as suggested for the L, M and O intermediates based on time-resolved FTIR18 and resonance Raman spectroscopy32. After 20 ms, a definite conclusion concerning the retinal isomer is difficult. The extrapolated maps suggest that a fraction of the retinal molecules may have already re-isomerized to the all-trans conformation, while it is still bent sideways.
Extended Data Fig. 4 Experimental setup and evolution of structural changes over time.
a, KR2 crystals were extruded in a stream of LCP from a high-viscosity injector. At the interaction point, the crystals were pumped with 575-nm femtosecond laser pulses before probing for structural changes with near parallel 12-keV XFEL pulses arriving from SwissFEL with a specific time delay Δt. The diffraction patterns were collected with a Jungfrau 16 M detector in a series of four light-activated patterns and one dark pattern, for which the visible pump laser was blocked. To reduce background through diffuse scattering of XFEL radiation in air, the experimental chamber was pumped down to 100–200 mbar while the residual air was replaced by helium. b, The evolution of structural changes in KR2 over time can be followed in a matrix of r.m.s.d. between all protein and retinal atoms (total of 2,703) in individual KR2 structural snapshots. The numerical r.m.s.d. values have been determined using the program pymol and are coloured in a blue–white–red gradient. The black boxes highlight time delays at which we combined data based on manual inspection of electron density maps and the evolution of photo intermediates in KR2 crystals determined by TR-IR spectroscopy. The approach was inspired by a recently published tool for visualizing protein motions in time-resolved crystallography66 but relies on refined atom positions instead of electron density changes. c, Difference electron density maps (Fo(light) − Fo(dark), negative density in gold and positive density in blue, contoured at 4σ and shown together with the KR2 resting state) obtained at the indicated time delays. The first panel is included as a control and shows difference electron density obtained from 50,000 patterns collected with laser off and 100,000 images from dark patterns obtained using the four light/one dark cycle used during TR-SFX data collection. For orientation, important residues discussed in the main text are shown as sticks.
Extended Data Fig. 5 Comparison of monomeric and pentameric KR2 structures.
Overall the structures at neutral pH reported in this work (left), in Kato et al.2 (middle) and in Kovalev et al.20 (right) are very similar (r.m.s.d. of Cα atoms of 0.30 and 0.72 Å, respectively) with the positions of residues and hydrogen bonding pattern (lower insets, black dotted lines, defined as distance ≥3.2 Å) in the binding pocket well preserved. Depending on conditions, the pocket in the pentameric structure can adopt the extended conformation shown. Here N112 is rotated out into the interaction interface between two KR2 protomers (coloured red) and the space is filled with water molecules. As the pentameric resting state can adopt a more open conformation in the SB region3, we cannot exclude the possibility that the electrostatic gate does not fully open in our crystals formed from monomeric KR2 (see main text). However, an unpublished steady-state structure of pentameric KR2 under continuous illumination (PDB 6XYT, Gordeliy group, IBS Grenoble) shows N112 rotated back into the binding pocket in a conformation very similar to our time-resolved millisecond states. As the retinal is modelled in the trans configuration, the 6XYT structure may represent a later intermediate compared to what we observe. The opening along the retinal (calculated by the program Caver39) in our 30 μs + 150 μs structure is 1.4 Å, which is very close to the 1.9 Å needed for a sodium ion to pass. Stable structural intermediates that accumulate in time-resolved studies of molecule ensembles do not necessarily reveal all functional states33 and it seems reasonable that a gate should form only when sodium is in close proximity to the SB. This would allow fast transfer of sodium to the extracellular side upon deprotonation. The gate would then collapse immediately upon sodium binding on the extracellular side, coinciding with reprotonation of the SB. Such a mechanism would efficiently prevent sodium back leakage. Differences in the position of N112 between the monomeric and pentameric resting states could furthermore explain the long-range effects of mutations in the oligomerization interface on the photocycle17 and sodium pumping efficiency1,17,20.
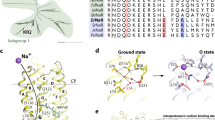
Extended Data Fig. 6 Comparison of ion binding sites in selected members of the rhodopsin family and QM/MM simulations.
a, Sodium binding site in Krokinobacter eikastus rhodopsin 2 (KR2, this work). b, Chloride binding site in the related bacterial pump Nonlabens marinus rhodopsin 3 (NM-R3, PDB 5G2867). c, Protonated SB in bacteriorhodopsin (bR, PDB 6RQP26). d, Chloride binding site in halorhodopsin (HR, PDB 1E1268). The retinal chromophore together with selected interactions and amino acid side chains are shown as dotted lines or sticks. Besides overall similarity in ion binding between bacteria and archea, the suggested binding site in KR2 is in line with quantum chemical calculation of absorbance changes upon sodium binding. e–g, The QM/MM-optimized geometry of the resting state (e) and the 1-ms structure with water (f) or sodium (g) bound to N112, D251. h, Comparison of the absorption shifts of the states measured in UV/Vis difference spectroscopy with the calculated QM/MM excitation wavelengths. Absolute values are given in parentheses. i, j, The extent and location of structural changes in KR2 at 20 ms (i) and bR at 15 ms (j) after activation (blue-to-red gradient and ribbon width indicate r.m.s.d. to the resting state, bR coordinates taken from previous work26). The light-driven pumping against a concentration gradient is achieved with smaller conformational changes in the order of 1–2 Å in sodium pumping KR2 compared to the more elusive protons pumped by bR24,26. The translocation path for sodium and protons (arrows), however, is similar for both proteins and includes three critical sites (see reviews on bR structural dynamics69 and activation mechanism70). Close to the water-filled cavity on the cytoplasmic side of KR2, Q123 of the NDQ motif is the most likely location for where the sodium ion loses its water coordination shell. The position is analogous to D96 of the DTD motif in bR, which is the primary donor for reprotonation at the end of the pumping cycle. The second critical site is formed by D116, N112 and D251, which correspond to D85, T89 and D212 in bR and coordinate sodium and proton transport in the SB region. The role of R109 in switching from water to sodium binding in KR2 is analogous in position and function to that of R82, which regulates the transfer of protons towards the release group in bR. The proton release group in bR is formed by E194 and E204, with the position overlapping well with the second sodium binding site between E11, N106 and E160 at the extracellular side of KR2. Beside these similarities, the sequence of events is clearly different between the two outward ion pumps. Light-driven pumping in bR starts with (1) a prebound proton followed by (2) release and (3) reloading. In KR2 the process is shifted in sequence with (1) entry, (2) binding and (3) release with a corresponding adaptation of the photocycle intermediates. The similar ion binding modes between bacteria and archea, together with how retinal isomerization is used to drive them, provides a notable example of the evolutionary economy of nature. The adaptation of this common leitmotif is particularly interesting in the case of KR2, where substrate binding has been shifted from the stable resting state into a transient intermediate.
Supplementary information
Video 1: Mechanistic Overview
The video shows the transition between six structural intermediates: resting state, 800fs+2 ps, 1ns+16ns, 30μs+150μs, 1ms and 20ms. The first step illustrates the absorption of a photon by the retinal chromophore which leads to trans-cis isomerization within a few hundred femtoseconds. Light energy is released when retinal tilts and pushes against V117 in nanoseconds, transmitting early changes along helix C. In the microseconds, the proton from the retinal SB is transferred to D116 leading to charge neutralization and opening of an electrostatic gate. Together with the relocation of helix C and G, this opens a pathway allowing w406 to be replaced by sodium. In the early milliseconds, the transported ion is ligated by N112 and D251 while the SB is reprotonated to prevent back leakage towards the intracellular side. In a final step, rearrangements of helix D forms a second binding site between E11, N106 and E160, from where sodium is released into the extracellular medium.
Video 2: High-speed jetting test of KR2 crystal preparations
Continuous and smooth extrusion of crystals is critical for a TR-SFX experiment. Monitoring the extrusion using a high-speed camera setup as described[40] allows efficient data collection. This time-lapse video shows the extrusion behaviour for a typical KR2 crystal preparation.
Rights and permissions
About this article
Cite this article
Skopintsev, P., Ehrenberg, D., Weinert, T. et al. Femtosecond-to-millisecond structural changes in a light-driven sodium pump. Nature 583, 314–318 (2020). https://doi.org/10.1038/s41586-020-2307-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2307-8
This article is cited by
-
Absolute and non-invasive determination of the electron bunch length in a free electron laser using a bunch compressor monitor
Scientific Reports (2024)
-
A subgroup of light-driven sodium pumps with an additional Schiff base counterion
Nature Communications (2024)
-
Influence of pump laser fluence on ultrafast myoglobin structural dynamics
Nature (2024)
-
Neurotoxin-Derived Optical Probes for Biological and Medical Imaging
Molecular Imaging and Biology (2023)
-
A multi-reservoir extruder for time-resolved serial protein crystallography and compound screening at X-ray free-electron lasers
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.