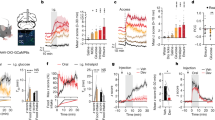
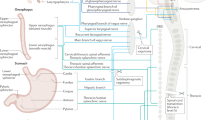
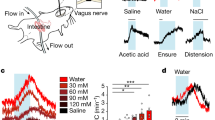
Abstract
Mechanosensory feedback from the digestive tract to the brain is critical for limiting excessive food and water intake, but the underlying gut–brain communication pathways and mechanisms remain poorly understood1,2,3,4,5,6,7,8,9,10,12. Here we show that, in mice, neurons in the parabrachial nucleus that express the prodynorphin gene (hereafter, PBPdyn neurons) monitor the intake of both fluids and solids, using mechanosensory signals that arise from the upper digestive tract. Most individual PBPdyn neurons are activated by ingestion as well as the stimulation of the mouth and stomach, which indicates the representation of integrated sensory signals across distinct parts of the digestive tract. PBPdyn neurons are anatomically connected to the digestive periphery via cranial and spinal pathways; we show that, among these pathways, the vagus nerve conveys stomach-distension signals to PBPdyn neurons. Upon receipt of these signals, these neurons produce aversive and sustained appetite-suppressing signals, which discourages the initiation of feeding and drinking (fully recapitulating the symptoms of gastric distension) in part via signalling to the paraventricular hypothalamus. By contrast, inhibiting the same population of PBPdyn neurons induces overconsumption only if a drive for ingestion exists, which confirms that these neurons mediate negative feedback signalling. Our findings reveal a neural mechanism that underlies the mechanosensory monitoring of ingestion and negative feedback control of intake behaviours upon distension of the digestive tract.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
All custom codes are available from the corresponding author upon reasonable request.
References
Schwartz, G. J. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition 16, 866–873 (2000).
Cummings, D. E. & Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Invest. 117, 13–23 (2007).
Umans, B. D. & Liberles, S. D. Neural sensing of organ volume. Trends Neurosci. 41, 911–924 (2018).
Andermann, M. L. & Lowell, B. B. Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 (2017).
Gizowski, C. & Bourque, C. W. The neural basis of homeostatic and anticipatory thirst. Nat. Rev. Nephrol. 14, 11–25 (2018).
Miller, N. E. Experiments on motivation. Science 126, 1271–1278 (1957).
Salet, G. A. M., Samsom, M., Roelofs, J. M. M., van Berge Henegouwen, G. P., Smout, A. J. P. M. & Akkermans, L. M. A. Responses to gastric distension in functional dyspepsia. Gut 42, 823–829 (1998).
Powley, T. L. & Phillips, R. J. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol. Behav. 82, 69–74 (2004).
Eisen, S., Davis, J. D., Rauhofer, E. & Smith, G. P. Gastric negative feedback produced by volume and nutrient during a meal in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1201–R1214 (2001).
Berthoud, H.-R. Vagal and hormonal gut–brain communication: from satiation to satisfaction. Neurogastroenterol. Motil. 20 (Suppl 1), 64–72 (2008).
Zimmerman, C. A., Leib, D. E. & Knight, Z. A. Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 18, 459–469 (2017).
Augustine, V., Lee, S. & Oka, Y. Neural control and modulation of thirst, sodium appetite, and hunger. Cell 180, 25–32 (2020).
Contreras, R. J., Beckstead, R. M. & Norgren, R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J. Auton. Nerv. Syst. 6, 303–322 (1982).
Saper, C. B. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 25, 433–469 (2002).
Palmiter, R. D. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293 (2018).
Ryan, P. J., Ross, S. I., Campos, C. A., Derkach, V. A. & Palmiter, R. D. Oxytocin-receptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nat. Neurosci. 20, 1722–1733 (2017).
McKinley, M. J. & Johnson, A. K. The physiological regulation of thirst and fluid intake. Physiology 19, 1–6 (2004).
Carter, M. E., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114 (2013).
Schwartz, G. J., McHugh, P. R. & Moran, T. H. Integration of vagal afferent responses to gastric loads and cholecystokinin in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 261, R64–R69 (1991).
Karimnamazi, H., Travers, S. P. & Travers, J. B. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res. 957, 193–206 (2002).
Grundy, D. & Scratcherd, T. in Comprehensive Physiology (ed. Terjung, R.) https://doi.org/10.1002/cphy.cp060116 (2011).
Williams, E. K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Garfield, A. S. et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 18, 863–871 (2015).
Li, C. et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 29, 681–694.e5 (2019).
Li, M. M. et al. The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102, 653–667.e6 (2019).
Kim, J. et al. Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat. Neurosci. 22, 576–585 (2019).
Barik, A., Thompson, J. H., Seltzer, M., Ghitani, N. & Chesler, A. T. A brainstem–spinal circuit controlling nocifensive behavior. Neuron 100, 1491–1503.e3 (2018).
Burnett, C. J. et al. Hunger-driven motivational state competition. Neuron 92, 187–201 (2016).
Cai, H., Haubensak, W., Anthony, T. E. & Anderson, D. J. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 17, 1240–1248 (2014).
Beutler, L. R. et al. Dynamics of gut–brain communication underlying hunger. Neuron 96, 461–475.e5 (2017).
Su, Z., Alhadeff, A. L. & Betley, J. N. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 21, 2724–2736 (2017).
Bai, L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143.e23 (2019).
Han, W. et al. A neural circuit for gut-induced reward. Cell 175, 665–678.e23 (2018).
de Lartigue, G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J. Physiol. 594, 5791–5815 (2016).
Kim, E. J., Jacobs, M. W., Ito-Cole, T. & Callaway, E. M. Improved monosynaptic neural circuit tracing using engineered rabies virus glycoproteins. Cell Rep. 15, 692–699 (2016).
Miyamichi, K. et al. Dissecting local circuits: parvalbumin interneurons underlie broad feedback control of olfactory bulb output. Neuron 80, 1232–1245 (2013).
Guo, Z. V. et al. Procedures for behavioral experiments in head-fixed mice. PLoS ONE 9, e88678 (2014).
Namboodiri, V. M. K. et al. Single-cell activity tracking reveals that orbitofrontal neurons acquire and maintain a long-term memory to guide behavioral adaptation. Nat. Neurosci. 22, 1110–1121 (2019).
Wickersham, I. R., Finke, S., Conzelmann, K.-K. & Callaway, E. M. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods 4, 47–49 (2007).
Loukas, M. et al. A review of the thoracic splanchnic nerves and celiac ganglia. Clin. Anat. 23, 512–522 (2010).
Lerner, T. N. et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015).
Wang, G. & Fowler, S. C. Effects of haloperidol and clozapine on tongue dynamics during licking in CD-1, BALB/c and C57BL/6 mice. Psychopharmacology 147, 38–45 (1999).
Marowitz, L. A. & Halpern, B. P. The effects of environmental constraints upon licking patterns. Physiol. Behav. 11, 259–263 (1973).
Rossi, M. A. & Yin, H. H. Elevated dopamine alters consummatory pattern generation and increases behavioral variability during learning. Front. Integr. Neurosci. 9, 37 (2015).
McConnell, E. L., Basit, A. W. & Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 60, 63–70 (2008).
Casteleyn, C., Rekecki, A., Van der Aa, A., Simoens, P. & Van den Broeck, W. Surface area assessment of the murine intestinal tract as a prerequisite for oral dose translation from mouse to man. Lab. Anim. 44, 176–183 (2010).
Stujenske, J. M., Spellman, T. & Gordon, J. A. Modeling the spatiotemporal dynamics of light and heat propagation for in vivo optogenetics. Cell Rep. 12, 525–534 (2015).
Susaki, E. A. et al. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat. Protoc. 10, 1709–1727 (2015).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Acknowledgements
We acknowledge B. K. Lim for the rabies viral vectors; the GENIE Program and the Janelia Farm Research Campus for GCaMP6 materials; T. J. Davidson and K. Deisseroth for help with the fibre photometry setup; H. Lu and U. Oh for help with targeting peripheral ganglia; P. Zhou for help with two-photon image analysis; and Olympus Korea Core Facility of College of Pharmacy of Seoul National University for two-photon microscopy setup. We are grateful to all members of the S.-Y.K. laboratory for helpful discussions, and A. Adhikari, H. Lee, M. E. Carter, J.-W. Sohn and G. S. B. Suh for comments on the manuscript. This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIP) (no. 2016R1C1B2007319 and no. 2016R1A4A1010796), grants of the Korea Health Technology R&D Project from the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant numbers: HI15C2887 and HI17C2665), Human Frontier Science Program (RGY0064/2017) and Creative-Pioneering Researchers Program of Seoul National University (SNU).
Author information
Authors and Affiliations
Contributions
D.-Y.K., G.H., M.K. and S.-Y.K. designed the project, interpreted the data and wrote the paper with input from all authors. D.-Y.K., G.H. and M.K. performed all experiments with contributions from H.K., J.A.J., H.-K.K., S.J., M.A., B.H.A., M.L. and G.J.S. H.-E.P. and J.H.P. established the fibre photometry and wrote the code for analysis. J.W.L. contributed to two-photon imaging. S.-Y.K. supervised all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Ivan E. de Araujo, Michael Krashes and Yuki Oka for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 FOS expression pattern in the parabrachial nucleus following water intake, and overlap of Pdyn and other neuronal markers in the parabrachial nucleus.
a, We began by probing a marker for neurons of the parabrachial nucleus that signal water intake, as an index of general ingestion. Water intake in dehydrated mice produced robust expression of FOS, a neural activity marker, in the dorsal lateral subnucleus of the parabrachial nucleus (dl), where the gene Pdyn (which encodes prodynorphin) is highly expressed. FOS immunofluorescence images (top) and the expression pattern of Pdyn from Allen Brain Atlas49 (bottom) along the AP axis of the parabrachial nucleus. Brain slices were obtained after ad libitum water access following 46-h water restriction. Image credit: Allen Institute. b–d, To visualize PBPdyn neurons, we bred a knock-in mouse line expressing Cre recombinase at the Pdyn locus (Pdyncre/+ mice) with Cre-dependent tdTomato reporter (Ai14) mice. Water-deprived Pdyncre/+ Ai14 mice or Pdyncre/+ mice injected with a Cre-inducible AAV carrying mCherry were given water access (W-D + water) or not (W-D) before the FOS analysis (b). Representative confocal images (c) and quantification (d) of the overlap between immunolabelled FOS+ and genetically labelled Pdyn+ neurons in the PBdl. The majority of tdTomato-expressing Pdyn+ neurons were FOS+ (about 72%), whereas few FOS+ neurons were observed in the PBdl of control mice that remained dehydrated (about 12% of Pdyn+ neurons). Most FOS+ PBdl neurons were also Pdyn+ (about 80%), indicating that Pdyn as a useful genetic handle for water-intake-activated neurons in the parabrachial nucleus. We also obtained consistent results in a separate set of experiments using mice in which PBPdyn neurons were fluorescently labelled using AAV vectors. There were smaller number of FOS+ neurons in the control mice (n = 146 and 7 neurons for experiments using Ai14 mice and AAV vectors, respectively) than in the experimental group (n = 492 and 1,002 neurons for experiments using Ai14 mice and AAV vectors, respectively). e, Representative images and quantification of multicolour fluorescence in situ hybridization experiments. Pdyn+ neurons are essentially glutamatergic (Slc17a6+) (>99%) (left), partially overlapping with fluid-intake-regulating Oxtr+ neurons16 (about 23%) (middle), but are separate from Calca+ neurons that have previously been implicated in noxious visceral signalling18 and meal termination15 (0%) (right). Scale bars, 100 μm (a), 10 μm (e), 50 μm (g, i). Data are presented as mean ± s.e.m. For statistics, see Supplementary Table 1. Abbreviations are defined in ‘Abbreviations’ in Methods.
Extended Data Fig. 2 PBPdyn neurons monitor ingestion using mechanosensory signals that arise from the upper digestive tract.
a, Fibre optic cannula targeting the parabrachial nucleus for fibre photometry were placed inside the red areas. b, c, Average calcium transients around the first (b) or the last lick (c) in every bout, showing time-locked responses of PBPdyn neurons to licking water (blue traces). The act of licking per se is not sufficient to activate PBPdyn neurons, as there was no response to empty-bottle licking (black). Inset top, average responses of all mice shown as auROC curve heat map. Inset bottom, peri-event plot of lick rate. d–g, Infusion of water or oil into the mouth, pharynx, oesophagus or stomach via oral gavage (d) induced strong activity in PBPdyn neurons (e). Average calcium responses in the first 5 s of infusion (f). The rate-controlled infusion of water or silicone oil evoked comparable responses in PBPdyn neurons, contrary to the uncontrolled voluntary consumption of water and oil (Fig. 1e), consistent with the intake-rate-dependency of the responses (Fig. 1f). Intragastric water infusion with longer intervals reveals the return of the activity to the baseline (g). h, PBPdyn neurons robustly responded to oral delivery of a small water drop (10 μl), which would not immediately flow beyond the mouth. i, j, Intragastric air infusion via oral gavage (i) or catheter (j) strongly activated PBPdyn neurons. k, Intragastric water infusion via oral gavage in anaesthetized mice also activated PBPdyn neurons. l, Local distension of the stomach wall using a blunt probe in anaesthetized mice robustly activated PBPdyn neurons. m, n, PBPdyn neurons did not significantly respond to the balloon-mediated distension of the duodenum (m) or proximal colon (n). o–r, Various test solutions were delivered to the mouth of head-fixed mice (o). PBPdyn neurons exhibited comparable responses to the intake of solutions with different taste (p), osmolality (q) and temperature (r). Insets show average calcium responses in the first 15 s of injection. Dotted lines indicate the onset and offset of infusion. Data are mean ± s.e.m. *P < 0.05; **P < 0.01, ****P < 0.0001. n.s., not significant. For statistics, see Supplementary Table 1.
Extended Data Fig. 3 Gastric and intestinal distension following air infusion, balloon inflation and ingestion.
a, b, Representative photographs of the stomach of an anaesthetized mouse before and after intragastric air infusion via oral gavage (a) or gastric catheter (b). For oral gavage, 1 ml of air was injected over 1 s, and then after 3 s the gavage needle was slowly withdrawn. Mice were rapidly euthanized at the indicated time points, the stomach was exposed and the pictures were taken. For the injection via gastric catheter, 1 ml of air was injected over 10 s. The stomach remained visibly distended at least for 3 min in both cases. c, d, PBPdyn neurons respond more strongly to drinking than feeding, as the intake rate is higher for fluids than solids. Although solids are generally considered to distend the stomach more potently than liquids for the slower emptying into the intestine, gastric emptying occurs on the order of tens of minutes, whereas the activity of PBPdyn neurons evoked by ingestion is on the order of seconds. In accordance with this view, we found that ad libitum intake of water in water-deprived mice for 1 min led to larger distension of the stomach (c) than the intake of chow food in food-deprived mice for the same duration (d). e, f, Representative photographs of the balloon-implanted duodenum (e) and proximal colon (f) before and after the injection of 50 μl saline into the balloon, showing noticeable distension.
Extended Data Fig. 4 Control recording and manipulation experiments for PBPdyn.
a–c, Activity of PBPdyn neurons was recorded during the open field test (a). Activities of PBPdyn neurons did not correlate with velocity (b), and were not significantly different regardless of whether mice were in the centre zone or in the periphery (c). d, Mice were placed on an elevated plus maze, in which open arms represent an anxiogenic environment. Activities of PBPdyn neurons were not significantly different regardless of whether mice were in the open or closed arms. e, PBPdyn neurons were not activated by gentle stroking of the dorsum of mice. Peri-event plot of average calcium transients and bar plot of average normalized ΔF/F before and during stroking are shown. f, PBPdyn neurons did not respond to touching of the whisker. g, PBPdyn neurons were not responsive to sensory detection of peanut butter. h, Head-fixed, water-deprived mice received 20 tones that co-terminated with water delivery, for 4 days. Average responses of PBPdyn neurons across training days, showing consistent and robust responses to water delivery (dark blue shaded box) but no responses to the auditory cue (grey shaded box). i, Optogenetic stimulation of PBPdyn neurons did not affect social interaction time with a juvenile stranger mouse. j, Chemogenetic stimulation of PBPdyn neurons did not alter time spent in the centre zone, indicating no change in anxiety-like behaviours. Data are presented as mean ± s.e.m. n.s., not significant. For statistics, see Supplementary Table 1.
Extended Data Fig. 5 Activity of PBPdyn neurons is not modulated by the thirst state.
a, Water was directly injected into the mouth, pharynx, oesophagus or stomach of dehydrated or euhydrated mice via oral gavage. b, c, Peri-event plot of average calcium transients (b) and average normalized calcium responses in the first 5 s of injection (c). Regardless of whether mice were dehydrated or euhydrated, PBPdyn neurons were robustly activated by water infused into any part of the upper digestive tract. d, e, The average calcium activity of PBPdyn neurons was not different before and after the thirst-inducing hypertonic saline injection (d) or 24-h water deprivation (e). Data are presented as mean ± s.e.m. *P < 0.05; **P < 0.01; ****P < 0.0001. n.s., not significant. For statistics, see Supplementary Table 1.
Extended Data Fig. 6 Mapping inputs to PBPdyn neurons.
a, Identification of monosynaptic inputs to PBPdyn neurons using engineered rabies virus. Representative confocal images showing the input regions. b–e, Engineered rabies-virus-mediated identification of monosynaptic inputs to the rostral NTS (b) and the Pr5 neurons that project to PBPdyn neurons (d). Representative images showing labelled neurons in the geniculate ganglion projecting to the rostral NTS neurons that project to PBPdyn neurons (c) and neurons in the ipsilateral trigeminal ganglion projecting to the Pr5 neurons that project to PBPdyn neurons (e). f–i, Total subdiaphragmatic vagotomy (SDx) (f) abolished the response of PBPdyn neurons to intragastric water injection via oral gavage (g), but thoracic level 5 spinal transection (T5x) (h) did not (i). j, Validation of subdiaphragmatic vagotomy. Following the intraperitoneal injection of the retrograde neural tracer Fluorogold, many labelled vagal motor neurons were found in the dorsal motor complex (DMX) of the sham control, but not in the vagotomized, mice. k, Validation of spinal transection. Following the injection of the retrograde neural tracer Retrobeads (Red RetroBeads; Lumafluor) into the parabrachial nucleus, many labelled neurons were found in the spinal cord of sham control, but not in the spinal-cord-transected, mice. l, Both electrophysiological and calcium imaging studies have previously demonstrated that vagal afferents that respond to gastric distension can be activated by CCK, a gut hormone released after a meal19,22. If PBPdyn neurons receive gastric distension signals via mechanosensory vagal fibres, CCK treatment should also activate the PBPdyn population. Indeed, intraperitoneal CCK injection strongly activated PBPdyn neurons. Scale bars, 200 μm for (k), 100 μm (a, c, e, j). Abbreviations are defined in ‘Abbreviations’ in Methods.
Extended Data Fig. 7 Activating PBPdyn neurons suppresses ingestion by reducing bout number rather than bout duration, mimicking the symptoms of gastric distension.
a, Saline control experiment for the experiment shown in Fig. 4b. b, Optogenetic stimulation of PBPdyn neurons. c, Mice were subjected to a two-bottle test. d, In dehydrated mice, optogenetic stimulation of PBPdyn neurons significantly suppressed water intake and tended to inhibit hypertonic saline intake. e, In euhydrated mice, no effect was observed. f, In salt-depleted mice, the same manipulation significantly decreased hypertonic saline intake and tended to inhibit water intake. g, h, The suppression in water intake shown in d was not driven by reduced bout duration (g) but instead by decreased bout number (h). i, Food-deprived mice were offered food without water to induce prandial thirst. Stimulating PBPdyn neurons suppressed water intake and tended to inhibit hypertonic saline intake in mice with prandial thirst. j, Ad libitum-fed, euhydrated mice were offered free access to sucrose solution (10%). Lick-paired optogenetic stimulation of PBPdyn neurons suppressed the intake of sucrose solution. k, Optogenetic stimulation of PBPdyn neurons in euhydrated mice elicited a trend for avoidance in real-time place preference test (RTPT). l, Water-deprived mice received intragastric infusion of 1 ml of air (air) or nothing (sham) via oral gavage, and then were provided with free access to water. m, Intragastric air infusion significantly increased the latency to the first lick. n–p, In the first 5 min, The air group consumed significantly smaller amount of water compared with the sham group (n). This was driven by decreased bout number (o) and not by bout duration (p). q–s, By 20 min from the onset of water intake, the total amount of consumed water became comparable between the two groups (q). The air group exhibited a significantly smaller bout number (r) and a trend towards longer bout duration (s). Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. n.s., not significant. For statistics, see Supplementary Table 1.
Extended Data Fig. 8 Projections of PBPdyn neurons and optogenetic stimulation of the projection to the paraventricular hypothalamus.
a, Anterograde tracing for visualizing target structures using synaptophysin–mRuby fusion protein. Major PBPdyn-neuron projection sites are indicated. Representative confocal images showing the parabrachial nucleus injection site and target structures. Green, GFP; black, synaptophysin–mRuby. b–g, Optogenetic stimulation of PBPdyn neuron projections to the paraventricular hypothalamus (b) activates postsynaptic paraventricular hypothalamus neurons, as indicated by increased Fos expression in the paraventricular hypothalamus (c). Yellow, Fos; blue, DAPI. This manipulation suppressed the intake of both water (d) and food (e) and induced avoidance in an RTPT (f). Pre-stimulation of the PBPdyn neuron projections to the paraventricular hypothalamus for 30 min before providing access to water elicited sustained inhibition in drinking behaviour in dehydrated mice for at least 10 min (g). h–k, Optogenetic stimulation of PBPdyn neuron projections to the subfornical organ (h) did not affect the intake of water (i) or food (j), but induced avoidance in an RTPT (k). Data are presented as mean ± s.e.m. *P < 0.05, ***P < 0.001, n.s., not significant. For statistics, see Supplementary Table 1. Scale bars, 100 μm. Abbreviations are defined in ‘Abbreviations’ in Methods.
Extended Data Fig. 9 Summary model.
PBPdyn neurons monitor the intake of both fluids and solids using mechanosensory distension signals from the upper digestive tract, which are transmitted via the cranial nerve pathways (with the vagus nerve conveying the gastric distension signals). The mechanosensory signals from distinct parts along the digestive tract probably converge at the parabrachial nucleus, such that individual PBPdyn neurons represent integrated mechanosensory signals from all parts of the upper digestive tract. In turn, PBPdyn neurons transmit sustained appetite-suppressing signals that deter the initiation (but not the maintenance) of ingestive behaviours to the downstream areas (including the paraventricular hypothalamus), to limit excessive feeding and drinking as a negative feedback. Abbreviations are defined in 'Abbreviations' in Methods.
Supplementary information
Supplementary Table
Supplementary Table 1 contains detailed descriptions of the sample sizes, statistical analyses that were used in this study.
Rights and permissions
About this article
Cite this article
Kim, DY., Heo, G., Kim, M. et al. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580, 376–380 (2020). https://doi.org/10.1038/s41586-020-2167-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2167-2
This article is cited by
-
A spatially-resolved transcriptional atlas of the murine dorsal pons at single-cell resolution
Nature Communications (2024)
-
Brain regulation of gastric dysfunction induced by stress
Nature Metabolism (2023)
-
Two-generation exposure to a high-fat diet induces the change of salty taste preference in rats
Scientific Reports (2023)
-
Gut feelings: mechanosensing in the gastrointestinal tract
Nature Reviews Gastroenterology & Hepatology (2022)
-
Sensory representation and detection mechanisms of gut osmolality change
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.