Abstract
Species that propagate by sexual reproduction actively guard against the fertilization of an egg by multiple sperm (polyspermy). Flowering plants rely on pollen tubes to transport their immotile sperm to fertilize the female gametophytes inside ovules. In Arabidopsis, pollen tubes are guided by cysteine-rich chemoattractants to target the female gametophyte1,2. The FERONIA receptor kinase has a dual role in ensuring sperm delivery and blocking polyspermy3. It has previously been reported that FERONIA generates a female gametophyte environment that is required for sperm release4. Here we show that FERONIA controls several functionally linked conditions to prevent the penetration of female gametophytes by multiple pollen tubes in Arabidopsis. We demonstrate that FERONIA is crucial for maintaining de-esterified pectin at the filiform apparatus, a region of the cell wall at the entrance to the female gametophyte. Pollen tube arrival at the ovule triggers the accumulation of nitric oxide at the filiform apparatus in a process that is dependent on FERONIA and mediated by de-esterified pectin. Nitric oxide nitrosates both precursor and mature forms of the chemoattractant LURE11, respectively blocking its secretion and interaction with its receptor, to suppress pollen tube attraction. Our results elucidate a mechanism controlled by FERONIA in which the arrival of the first pollen tube alters ovular conditions to disengage pollen tube attraction and prevent the approach and penetration of the female gametophyte by late-arriving pollen tubes, thus averting polyspermy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Takeuchi, H. & Higashiyama, T. A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol. 10, e1001449 (2012).
Zhong, S. et al. Cysteine-rich peptides promote interspecific genetic isolation in Arabidopsis. Science 364, eaau9564 (2019).
Li, C., Wu, H. M. & Cheung, A. Y. FERONIA and her pals: functions and mechanisms. Plant Physiol. 171, 2379–2392 (2016).
Duan, Q. et al. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 5, 3129 (2014).
Dresselhaus, T. & Franklin-Tong, N. Male–female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol. Plant 6, 1018–1036 (2013).
Higashiyama, T. & Yang, W. C. Gametophytic pollen tube guidance: attractant peptides, gametic controls, and receptors. Plant Physiol. 173, 112–121 (2017).
Wu, H., de Graaf, B., Mariani, C. & Cheung, A. Y. Hydroxyproline-rich glycoproteins in plant reproductive tissues: structure, functions and regulation. Cell. Mol. Life Sci. 58, 1418–1429 (2001).
Cheung, A. Y., Wang, H. & Wu, H. M. A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82, 383–393 (1995).
Escobar-Restrepo, J. M. et al. The FERONIA receptor-like kinase mediates male–female interactions during pollen tube reception. Science 317, 656–660 (2007).
Duan, Q., Kita, D., Li, C., Cheung, A. Y. & Wu, H.-M. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl Acad. Sci. USA 107, 17821–17826 (2010).
Punwani, J. A., Rabiger, D. S. & Drews, G. N. MYB98 positively regulates a battery of synergid-expressed genes encoding filiform apparatus localized proteins. Plant Cell 19, 2557–2568 (2007).
Beale, K. M., Leydon, A. R. & Johnson, M. A. Gamete fusion is required to block multiple pollen tubes from entering an Arabidopsis ovule. Curr. Biol. 22, 1090–1094 (2012).
Kasahara, R. D. et al. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr. Biol. 22, 1084–1089 (2012).
Maruyama, D. et al. Rapid elimination of the persistent synergid through a cell fusion mechanism. Cell 161, 907–918 (2015).
Feng, W. et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 28, 666–675.e5 (2018).
Peaucelle, A., Braybrook, S. & Höfte, H. Cell wall mechanics and growth control in plants: the role of pectins revisited. Front. Plant Sci. 3, 121 (2012).
Hou, W.-C., Chang, W.-H. & Jiang, C.-M. Qualitative distinction of carboxyl group distribution in pectins with ruthenium red. Bot. Bull. Acad. Sin. 40, 115–119 (1999).
Pattathil, S. et al. A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 153, 514–525 (2010).
Peaucelle, A. et al. Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr. Biol. 18, 1943–1948 (2008).
Wolf, S., Mravec, J., Greiner, S., Mouille, G. & Höfte, H. Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr. Biol. 22, 1732–1737 (2012).
Besson-Bard, A., Pugin, A. & Wendehenne, D. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 59, 21–39 (2008).
Domingos, P., Prado, A. M., Wong, A., Gehring, C. & Feijo, J. A. Nitric oxide: a multitasked signaling gas in plants. Mol. Plant 8, 506–520 (2015).
Planchet, E. & Kaiser, W. M. Nitric oxide (NO) detection by DAF fluorescence and chemiluminescence: a comparison using abiotic and biotic NO sources. J. Exp. Bot. 57, 3043–3055 (2006).
Boavida, L. C., Borges, F., Becker, J. D. & Feijó, J. A. Whole genome analysis of gene expression reveals coordinated activation of signaling and metabolic pathways during pollen–pistil interactions in Arabidopsis. Plant Physiol. 155, 2066–2080 (2011).
Rasul, S. et al. Nitric oxide production mediates oligogalacturonide-triggered immunity and resistance to Botrytis cinerea in Arabidopsis thaliana. Plant Cell Environ. 35, 1483–1499 (2012).
Takeuchi, H. & Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531, 245–248 (2016).
Zhang, X. et al. Structural basis for receptor recognition of pollen tube attraction peptides. Nat. Commun. 8, 1331 (2017).
Clausen, M. H., Willats, W. G. & Knox, J. P. Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr. Res. 338, 1797–1800 (2003).
Hruz, T. et al. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv. Bioinformatics 2008, 420747 (2008).
Huang, Y. C. et al. PECTIN METHYLESTERASE34 contributes to heat tolerance through its role in promoting stomatal movement. Plant Physiol. 174, 748–763 (2017).
Terrile, M. C. et al. Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J. 70, 492–500 (2012).
Prado, A. M., Porterfield, D. M. & Feijó, J. A. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 131, 2707–2714 (2004).
Wilkinson, J. Q. & Crawford, N. M. Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes NIA1 and NIA2. Mol. Gen. Genet. 239, 289–297 (1993).
Sanz, L. et al. Nitric oxide plays a role in stem cell niche homeostasis through its interaction with auxin. Plant Physiol. 166, 1972–1984 (2014).
Guo, F. Q., Okamoto, M. & Crawford, N. M. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302, 100–103 (2003).
Moreau, M., Lee, G. I., Wang, Y., Crane, B. R. & Klessig, D. F. AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J. Biol. Chem. 283, 32957–32967 (2008).
Okuda, S. et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458, 357–361 (2009).
Qu, Z. et al. Proteomic quantification and site-mapping of S-nitrosylated proteins using isobaric iodoTMT reagents. J. Proteome Res. 13, 3200–3211 (2014).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Schiøtt, M. et al. A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl Acad. Sci. USA 101, 9502–9507 (2004).
Sheng, X. et al. Lead stress disrupts the cytoskeleton organization and cell wall construction during Picea wilsonii pollen germination and tube growth. Biol. Trace Elem. Res. 146, 86–93 (2012).
Chebli, Y., Kaneda, M., Zerzour, R. & Geitmann, A. The cell wall of the Arabidopsis pollen tube—spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol. 160, 1940–1955 (2012).
Leroux, C. et al. PECTIN METHYLESTERASE48 is involved in Arabidopsis pollen grain germination. Plant Physiol. 167, 367–380 (2015).
Ischebeck, T., Stenzel, I. & Heilmann, I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. Plant Cell 20, 3312–3330 (2008).
Pleskot, R. et al. Turnover of phosphatidic acid through distinct signaling pathways affects multiple aspects of pollen tube growth in tobacco. Front Plant Sci. 3, 54 (2012).
Smyth, D. R., Bowman, J. L. & Meyerowitz, E. M. Early flower development in Arabidopsis. Plant Cell 2, 755–767 (1990).
Chormova, D., Messenger, D. J. & Fry, S. C. Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion but not post-secretion. Plant J. 77, 534–546 (2014).
Nilesh, R. et al. Extraction of pectin from citrus fruit peel and use as natural binder in paracetamol tablet. Pharm. Lett. 4, 558–564 (2012).
Acknowledgements
We thank G. Drews for DD2p::DD2 (AtLURE1)-GFP seeds; M. Johnson for hap2/+ seeds; B. McClure for LAT52::tdTomato transformed Arabidopsis seeds; H. Hofte for 35S:PMEI5 seeds; a large number of undergraduates who helped with plant growth and maintenance over many years, and K. McNamara and T. Lichoulas, in particular, for contributing to protein purifications; P. Huesgen and R. Tomaino for advice, and the Harvard Medical School Talpin mass spectrometry facility for service; and Y.-j. Zou for the blue-dot assay shown in Extended Data Fig. 1a. M.-C.J.L. was partially supported by the Ministry of Science and Technology Overseas Project, 2013 Graduate Student Study Abroad Program; and L.E.G.V. was partially supported by a fellowship from Estancias Posdoctorales en el Extranjero, Vinculadas a la Consolidación de Grupos de Investigación y Fortalecimiento del Posgrado Nacional 2018. This work was supported by grants from the NSF (IOS-1147165, -1146941, -1645854 and MCB-1715764 to A.Y.C. and H.-M.W.). Mass spectrometry data were partially obtained at the University of Massachusetts Mass Spectrometry Center, a facility supported by the National Institutes of Health (S10OD010645); the plant growth facility was partially supported by NIFA/USDA, the Center for Agriculture, Food and the Environment, under project number MAS00525. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, USDA or NIFA. Confocal microscopy was performed in the Light Microscopy Facility and Nikon Center of Excellence at the Institute for Applied Life Sciences, University of Massachusetts with support from the Massachusetts Life Science Center.
Author information
Authors and Affiliations
Contributions
A.Y.C. and H.-M.W. designed the overall research plan and led the writing process; Q.D. and M.-C.J.L. participated in strategy and method development for their respective focus parts, and in the writing process. Q.D. performed pollen tube–ovule interaction experiments throughout the study, with contributions to the pectin aspect from D.K. until 2012. M.-C.J.L. contributed to pectin studies and performed the biochemical studies in NO–LURE interaction (since 2013); C.S.-W. contributed to co-supervising M.-C.J.L. as a PhD mentor during the early parts of this study (2013–2015). S.J.E. provided mass spectrometry service and contributed to mass spectrometry data analysis; S.S.J., F.-L.J.Y., R.Y., H.C. and L.E.G.-V. contributed to the FER–pectin–NO–LURE biochemical studies; A.N.F., H.C. and Q.D. carried out seedling studies. All authors participated in finalizing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks John Hancock, Hermanus Hofte and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
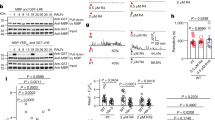
Extended Data Fig. 1 FER-regulated pollen tube–ovule interaction and pectin deposition in the filiform apparatus.
a, Blue-dot assay, an alternate method (from that in Fig. 1b) to show pollen tube targeting and penetration of ovules. β-GUS-expressing pollen grains (Pg, blue) were used to pollinate a wild-type pistil, allowing the pollen tube growth path to be visualized and demonstrated the prevalently 1:1 pollen tube:ovule ratio under normal pollination conditions. This result is typical of pollinated wild-type pistils, and it was repeated at least five times in the course of this study. b, FER promoter (FERp)::FER–GFP expression in a complemented fer-4 pistil, showing prominent localization of FER–GFP at the filiform apparatus region (arrows)4 as well as expression in sporophytic tissues, such as the integuments (In) and funiculus (F). For this study, the observation was repeated at least three times in independent preparations. c, Pollen tube reception defects induced by fer-4 mutation (pollen tube overgrowth inside the female gametophyte owing to non-rupture) and multiple pollen tube entrance phenotypes. d–f, RuR-detected pectin in ovules. d, Wild-type and fer-4 ovules showed developmental regulation and FER-dependent pectin deposition at the filiform apparatus or micropylar region (arrowheads). Floral stages followed that of previous publications4,46; stages 13–15 are the most receptive for pollination—at stage 16 ovules had enlarged, reflecting successful fertilization. e, Quantification of RuR-positive filiform apparatus region. f, FERp::FER–GFP complemented fer-4 mutation restored deposition of de-esterified pectin at the filiform apparatus, as it did the normal single pollen tube penetration phenotype (Extended Data Fig. 1c). g, Quantification of de-esterified pectin located at the filiform apparatus by JIM5 and M38 signal levels, following a previously described method4. Average signal intensity in equal areas of interest (dotted lines) at the filiform apparatus and in the synergid cells were compared. A filiform apparatus:synergid cell ratio of ≥1.2 was scored as JIM5- or M38-positive. h, JIM7 immunostaining of ovule methylesterified pectin. Arrows, filiform apparatus region. Image acquisition conditions were the same as those for JIM5 immunostaining (Fig. 1e). Signal was below detection, most probably because of the high solubility of the methylesterified polymers during the immunostaining procedure, which involved extensive washes. i, JIM5 immunostaining of wild-type and FERp::PMEI1–GFP (PMEI1ox) ovules. Overexpression of PMEI1 (Extended Data Fig. 2) reduced the localization of de-esterified pectin at the filiform apparatus. j, M38 immunostaining of wild-type, pme44, pme34 and PMEIox ovules. Reduced M38 and JIM5 signals correlated with augmented multiple pollen tube entry phenotype (Fig. 1g). Data shown are average ± s.d. n, pistil numbers (c, e, f, h–j); n, ovule numbers (g). Data are representative of three independent experiments. P values were obtained by two-tailed t-tests; numbers in data plots represent the number of ovules examined. Scale bar, 1 mm (a), 50 μm (d, g, h). Box plots: centre line, median; box limits, lower and upper quartiles; dots, individual data points; whiskers, highest and lowest data points.
Extended Data Fig. 2 Characterization of mutants deficient in de-esterified pectin.
a–d, Transfer (T-) DNA insertion mutants pme34-1 (Salk_062058)30 and pme44-1 (SALK_071362). a, c, Genomic maps with T-DNA insertions (triangles). b, d, PCR with reverse transcription for PME34 and PME44 mRNAs from seedlings (S) and flowers (F). Green arrows, primers for RT–PCR; black and red arrow pairs, primers for genotyping. e–g, Flowering wild-type, pme34-1, pme44-1 and PMEI1ox plants. Growth, flowering time, flower morphology and reproductive yields (silique sizes, arrows in f, g, and seed numbers) of these transgenic mutant plants were adequately normal relative to wild type, permitting reproductive studies. h–k, FERp::PMEI1–GFP expression and ovule morphology in PMEI1ox plants. PMEI1–GFP expression in seedlings and ovules paralleled that of FERp::FER–GFP4,10, including a most-prominent accumulation in the elongation zone of seedling roots (h), throughout the ovules and prominently at the filiform apparatus region (arrowheads) (i). Ovules appeared mostly normal, although higher level of autofluorescence was observed in the sporophytic tissue of some ovules (white arrows). When stained by aniline blue, most ovules from the plants used here appeared normal (j). Some ovules showed elevated callose deposition in the sporophytic as well as in the female gametophyte region (k), reflecting stress. Ovules with overaccumulation of callose are generally not penetrated by pollen tubes, so were excluded from these studies. Extended Data Figure 10a–c shows defects in a PMEI5-overexpression line20, precluding its use in these studies. l, Statistical comparison among mutants deficient in de-esterified pectin and transgenic plants. The levels of ovules penetrated by multiple pollen tubes in pme34, pme44 and PMEI1ox plants were not significantly different among them (bracket 1). Deficiency in de-esterified pectin among these mutant ovules was also not significantly different (Extended Data Fig. 1j). Attempts to compound their effect in a pme34 pme44 double mutant (arrowheads indicate a penetrating pollen tube doublet) did not result in a significantly higher multiple-tube penetration phenotype than the single mutant parents (bracket 2). Data for pme34, pme44 and PMEI1ox plants were from Fig. 1g. Data for pme44 and pme34 are average ± s.d. n, number of pistils. Results were representative of three independent experiments. P values were obtained by two-tailed t-tests; numbers in data plots denote the number of ovules examined. Box plots: centre line, median; box limits, lower and upper quartiles; dots, individual data points; whiskers, highest and lowest data points.
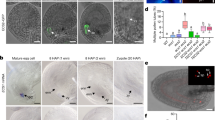
Extended Data Fig. 3 Ovular NO status in wild type and NO-deficient mutants.
a, Comparison of two methods of ovule NO quantification. Ovules from pollinated (16 h after pollination) and unpollinated wild-type pistils at the same developmental stage were examined. Images were acquired with identical conditions and a sampling of ovules is shown. In the direct scoring method, ovules were scored as positive (+) when signal at the filiform apparatus (arrow, filiform apparatus) was notably higher than in the synergid cell (right histogram). In the filiform-apparatus/synergid-cell signal-intensity-ratio method, average signal intensity of identical areas at the filiform apparatus and in the synergid cell (as background) was determined by ImageJ. A filiform apparatus synergid cell ratio of ≥1.2 was scored as + (left dot plot). The two methods gave comparable conclusions. Data are average ± s.d. n, number of ovules. P values were obtained by two-tailed t-tests. b, Full ovule images for Fig. 2b. The ejected pollen cytoplasm (red) marked the synergid cell, providing a clear spatial definition for NO located at the filiform apparatus (arrow). c, Comparison of filiform apparatus:synergid cell NO signal intensity in pollinated wild-type and fer-4 ovules (left dot plot) and direct scoring (right histogram). Results were comparable. Data (left dot plot) are average ± s.d. n, number of ovules. d, NO in wild-type, nia1 nia2 and noa1 mutant ovules. The results correlated reduced filiform-apparatus NO in these pollen-tube-penetrated ovules with elevated multiple pollen tube entrance (Fig. 2e). Data are average ± s.d. n, number of pistils. Numbers in plot denote the number of ovules examined. P values were obtained by two-tailed t-tests. Data are representative of three independent experiments. Scale bars, 50 μm. Box plots: centre line, median; box limits, lower and upper quartiles; dots, individual data points; whiskers, highest and lowest data points.
Extended Data Fig. 4 Quantification of pollinated pistil exudate.
a, Pollinated wild-type and fer-4 pistils. One anther of pollen was used per emasculated pistil. Aniline-blue staining showed comparable quantity and quality of pollen tube growth in both pistils, and the typical single pollen tube (arrowheads) exiting the wild-type transmitting track, bundled pollen tubes (arrows) exiting the fer-4 pistils (as seen in Fig. 1b, Extended Data Fig. 1a), and pollen tube overgrowth in fer-4 ovules (*). Pistillate exudate preparations were from 100 pistils, concentrated to 20 μl (see Methods for details). b, Dot blot quantification of PGA and pistil exudates. Top row, 2 μl of a concentration series of fragmented PGA was spotted. Bottom row, exudates equivalent to two (spot 2) and four (spot 4) pollinated wild-type pistils were applied to a membrane filter. JIM5 immunodetection signals approximated 60 ng of de-esterified pectin from 4 pistils (about 15 ng per pistil). Extended Data Figure 5b shows a PGA sample. c, JIM5 immunodetection of four pistillate exudates (1–4) as indicated. Two microlitres—equivalent to exudates from 10 pistils—was spotted on filter; PGA dots were prepared as described in b for dot blot quantification. JIM5 was used for immunodetection. Only exudate 2, from pollinated wild-type pistils, showed a notable level of JIM5-detected de-esterified pectin, approximating 125 ng (about 12.5 ng per pistil). A low level of JIM5 signal in sample 4 correlated with few aca9-1 pollen tubes in the pollinated pistils (Extended Data Fig. 10d, e). Four microlitres (that is, about 250 ng of JIM5-detected materials) of similarly prepared exudates was applied to ovule NO assays (Fig. 3b). On the basis of pistil dry weight (32 μg per pistil, averaged from batches of 25 dried pistils from flowers around stage 14), the pistillate exudate JIM5-detectable material recovery would be about 0.04%. These quantifications were, however, approximations; precise quantification of these reagents will need substantial further refinement.
Extended Data Fig. 5 De-esterified pectin induces FER-dependent NO production.
a, Exudates from pollinated wild-type, but not fer-4, pistils induced NO at the filiform apparatus region (arrows) of wild-type ovules. b, Sonicated PGA (100 μg) electrophoresed in a 26% polyacrylamide gel47. Lanes represent fractions eluted from a diethylaminoethyl column by the indicated salt gradient. The last fraction approximated PGA before sonication. Oligogalacturonic-acid-sized25 fragments would be ahead of the RuR-stained materials on the basis of comparison with a previous publication47. Active species for ovular NO induction remains to be determined. c, Representative quantification of PGA-induced NO response based on filiform apparatus:synergid cell signal ratio, as described in Extended Data Fig. 3a. Data plots summarize the quantification data (left dot plot) and data from direct scoring (right histogram), showing comparable results. Data are average ± s.d., n, number of ovules. d, cPTIO suppressed PGA induced accumulation of NO at the filiform apparatus. cPTIO was added together with the DAF-DA dye. Data are average ± s.d. n, number of pistils. e, PGA treatment of wild-type and fer-4 ovules showed NO in wild-type (arrow), but not fer-4, filiform apparatus. f, PGA treatment of wild-type, fer-4 and FER–GFP-complemented fer-4 seedling roots showed FER dependence for PGA-induced root NO. Under equal image acquisition conditions (but without added DAF-DA), FER–GFP signal was negligible (last data bar). Signals were quantified from equal areas of interest (white box). Data are average ± s.d., n, number of seedlings. P values were obtained by two-tailed t-tests. Scale bars, 50 μm. All observations were representative of three independent experiments. Box plots: centre line, median; box limits, lower and upper quartiles; dots, individual data points; whiskers, highest and lowest data points.
Extended Data Fig. 6 Effect of pectic fragments and NO on LURE1 properties.
a–c, Wide-field images of control, GSNO-, SNP- and PGA-treated LURE1–GFP-expressing ovules from unpollinated pistils. Data are average ± s.d. n, number of pistils. Representative of three independent experiments. P values were obtained by two-tailed t-tests; numbers in plots denote the number of ovules examined. d, SDS–PAGE of purified E.-coli-produced MBP, MBP–LURE1 (arrowheads) and the extracellular domain of its receptor PRK6 (His6–HA–PRK6(ecd)) (arrow). M, molecular mass markers. e, MBP–LURE1 attracts pollen tubes. Top, purified MBP–LURE1 was used in pollen tube attraction assays1,37 in semi-pollen tube growth cultures. Arrowheads, tips of pollen tubes at the time of bead application. Bottom, histogram shows MBP–LURE1 dose-dependent pollen-tube-attraction activity. Attraction efficiency was similar to that in a previous study1. Mock treatment (0) used MBP in gelatin beads. Scale bars, 100 μm. Data are representative of multiple independent protein preparations using a similar range of MBP–LURE1 concentrations. f–h, GSNO treatment of MBP–LURE1. f, MBP–LURE1 was mock-treated or incubated with GSNO before application for SDS–PAGE in the absence of β-mercaptoethanol, followed by Coomassie-blue staining. g, An immunoblot by anti-MBP antibody of an experiment similar to that in f. Arrow, double arrowheads and bracket indicate monomeric MBP–LURE1, dimer-sized and higher-molecular-weight forms, respectively. h, Comparison of GSNO treatment of MBP and MBP–LURE1 shows no notable effect on the molecular weight of MBP. Collectively comparable observations were made in at least six independent experiments. i, j, Dot blot assay for LURE1 interaction with its receptor PRK626,27. i, Equal volumes (1.5 μl) of increasing concentrations of HA–PRK6(ecd) were applied to filter in triplicate rows for interaction with MBP–LURE1, then processed for immunodetection of bound MBP–LURE1 by anti-MBP antibody. Ponceau-S-stained filter illustrates quantitative spotting of HA–PRK(ecd) to the membrane. Data plot (right) shows signal intensity from the bound MBP–LURE1. Data are averaged from the triplicate binding samples ± s.d., showing a Kd of about 1.21 ± 0.28 μM, approximating the previously reported affinity26. j, Dot blot assay for PRK6(ecd) interaction with control (0) and MBP–LURE1 treated with increasing concentration of GSNO. PRK6(ecd) on membrane was incubated with MBP–LURE1, and the interaction was detected by anti-MBP. Ponceau-S-stained filters confirmed quantitative spotting of PRK6(ecd). The result from the 12-μg bait blots (top row) is shown in Fig. 4c. k, Effect of DTT on GSNO-induced inhibition of MBP–LURE1 binding to HA–PRK6(ecd). MBP–LURE1 was preincubated with combinations of DTT and GSNO as shown, before application to an HA–PRK6(ecd) filter for binding. Ponceau-S-stained filters illustrate comparable HA–PRK6(ecd) applied to all filters. Results indicate reducing conditions mitigated GSNO inhibition. Collectively, similar observations were made in three independent experiments (i, j) or two for k; the triplicated and duplicated dots in i, k served as technical replicates. Box plots: centre line, median; box limits, lower and upper quartiles; dots, individual data points; whiskers, highest and lowest data points.
Extended Data Fig. 7 Analysis of LURE1 nitrosation.
a, Coomassie-blue-stained gel of typical MBP and MBP–LURE1 preparations used for nitrosation assays. b, Ponceau-S-stained protein blot for detection of TMT-labelled MBP–LURE1 (arrow); immunoblot is shown in Fig. 4e. c, Ponceau-S-stained protein blot for detection of TMT-labelled MBP–full-length LURE1 (arrow); immunoblot is shown in Fig. 4e. d, Coomassie blue-stained purified MBP–full-length LURE1 after TMT-labelling reaction (arrow). The recombinant protein with LURE1 signal peptide tended to break down during the labelling procedure, producing a lower band at the MBP molecular weight range. The MBP-full LURE1 protein bands indicated by the arrow were excised for mass spectrometry analysis shown in Fig. 4e, g, h. e, LC–MS/MS spectrum showing TMT–Cys58-containing peptides, and LURE1.2 amino acid sequence highlighting Cys58. f, LURE1p::LURE1–GFP localization in ovules from unpollinated (BP) or pollinated (AP) pistils for comparison with LURE1(C17A)–GFP localization (shown in Fig. 4i). As shown in Fig. 4a, LURE1–GFP typically located in the filiform apparatus in ovules from unpollinated pistils, and delocalized to the cytoplasm of synergid cells in ovules from pollinated pistils. Box plots: centre line, median; box limits, lower and upper quartiles; dots, individual data points; whiskers, highest and lowest data points.
Extended Data Fig. 9 A summary of FER-regulated pollen tube–ovule interaction.
The diagram summarizes previously published results on how FER mediates pollen tube rupture and sperm release to enable fertilization4 (steps 2 and 3 in the scheme), and results reported here on how FER affects two interconnected conditions at the filiform apparatus to prevent the penetration of ovules by supernumerary pollen tubes (steps 4 and 5 in the scheme). Solid lines reflect information that comes directly from experimental data; dashed lines reflect extrapolations from experimental data and other relevant information. Results present in this Article show that FER maintains a pistillate environment enriched in de-esterified pectin (Fig. 3a) (step 1 in the scheme), including at the filiform apparatus of the female gametophyte (Fig. 1e, f). Pollen tubes are attracted by female guidance cues to depart from their main growth axis to approach ovules, and are then guided by synergid-cell-produced chemoattractants (for example, LUREs) to the ovules to penetrate the female gametophyte1,5,6 (Fig. 1b,c, Extended Data Fig. 1b). In penetrating the filiform apparatus that is enriched in de-esterified pectin, the pollen tube that arrives first should continue to secrete cell-wall degradative enzymes—presumably producing pectic fragments in the vicinity, just as growth in the transmitting track produced these polymers (Fig. 3a, Extended Data Fig. 4). The application of pollinated pistillate exudate enriched in de-esterified pectin (Fig. 3b) and fragmented, commercially obtained de-esterified pectin (Fig. 3c, d) to ovules from unpollinated pistils, and the arrival of pollen tubes at ovules in pollinated pistils (Fig. 2a, b) (step 4 in the scheme), triggered NO accumulation at the filiform apparatus. Taken together with the observation that NO-deficient ovules also had elevated levels of multiple pollen tube entrance (Fig. 2e), these results are consistent with pectic fragments generated by pollen tube growth acting as a mediator to trigger NO accumulation to prevent the entrance of supernumerary pollen tubes (step 4 in the scheme). Our results demonstrate that NO does so by modifying LURE and disengaging LURE-mediated attraction of late-arriving pollen tubes to already penetrated ovules (steps 4 and 5 in the scheme) (Fig. 4, Extended Data Figs. 6, 7). Previously published results4, and the properties of de-esterified pectin, are consistent with an altered ovular environment before and after pollen tube rupture. This could be achieved (for example) by Ca2+ leakage from the degenerating synergid cell and Ca2+ released from the ruptured pollen tube. The elevated extracellular [Ca2+] would immediately mediate stiffening of the filiform apparatus that is rich in de-esterified pectin. The stiffened cell wall could then present a back-up blockade to prevent entrance by an errant, late-arriving pollen tube into an already penetrated female gametophyte (step 6 in the scheme).
Extended Data Fig. 10 Additional considerations.
a–c, CaMV35S::PMEI5-transformed plants are severely compromised in female fertility. a, Constitutive expression of PMEI5 severely affected plant development20. b, Flower organogenesis appeared normal, but pistil development was suppressed; at maturity, pistils were at most 1/3 of the normal size. Pollen production appeared normal. c, The stigmatic papillae of these plants were under-developed; pollinated stigma did not retain any pollen grains, reflecting their inability to support pollen germination to penetrate the stigmatic papillae. The aniline blue-stained ovules showed high levels of callose deposition, a symptom of stress. Even in normal pistils, ovules with ectopic callose accumulation do not attract pollen tubes (Extended Data Fig. 2j, k), precluding these PMEI5-overexpressing ovules from being used in ovule penetration studies. Very few seeds were produced from these PMEI5-overexpressing plants (about 10 μl of seeds from a full pot was a good yield under our growth condition). Similar observations were made in several plantings, as we obtained the seeds in 2014.d, e, aca9-1 pollinated pistils. d, Flowering aca9-1 plants produced many under-developed siliques (black); even elongated siliques (white arrows) had a substantially reduced number of fertilized ovules40. e, aca9-1 pollination of wild-type pistils. Aniline blue staining showed few elongating pollen tubes and their arrival at ovules (arrowheads), even at 30 h after pollination. Exudates from pistils pollinated by aca9-1 pollen showed a basal level of de-esterified pectin (Extended Data Fig. 4c). These observations are linked to the experiment presented in Extended Data Fig. 4; together, four independent preparations showed comparable results. f–i, NO as a gaseous agent to block the entrance of supernumerary pollen tubes. Observations below are included here to relate how findings from the present study might be linked to several topics that are not yet fully understood. f, Confocal images of mature ovules from unpollinated pistils expressing a moderate level of LURE1–GFP (top; a large majority of ovules in the transformed pistils), showing the typical filiform-apparatus (arrow) localization. Occasionally, an ovule overexpressed LURE1–GFP (bottom), showing the presence of the protein in the inner integuments (i) that envelope the micropylar (m) chamber. The working distance of the LURE1 gradient diffused from the filiform apparatus is not known, although histoimmunodetection suggests that it reaches the micropylar region1. NO, as a gaseous molecule, should diffuse readily and reach the micropylar region (and possibly beyond), although its working distance is unknown and difficult to determine. These observations are included for the consideration of plausible functional linkage between the two gradients that would be expected to exist in the system. g, LURE1–GFP localization at completion of pollination (20 h after pollination) in LURE1p::LURE1–GFP pistils by wild-type pollen. Typically fewer than 50% of the ovules showed notable GFP signal in the synergid cell (category 1) (Fig. 4a, b); others retained a weak synergid cell signal (category 2). A low percentage of ovules showed LURE1–GFP localization in both the filiform apparatus (arrow) and the synergid cell (category 3). When pollinated by pollen from hap2/+ mutant plant, the sperm from half of the applied pollen (hap2 pollen) was incompetent for fusion; therefore, half of the ovules were not fertilized12. A higher percentage of ovules from hap2/+ pollinated pistils showed category-3 localization, with notable synergid cell as well as filiform apparatus LURE1–GFP signal. h, Confocal image of a category-3 ovule. A maximum projection from 4 images (1 μm total thickness) (left) and a single optical section (right), showing LURE1–GFP localization in a synergid cell and across the filiform apparatus. It could be envisioned that category 3 ovules in hap2/+ pollinated pistils could be candidates for fertilization recovery (that is, having the propensity to salvage fertilization). In the hours after failed fertilization, NO induced by the arrival of the sterile hap2 pollen tube could have dissipated (for example, see data plot in Fig. 2c), allowing some level of secretion of newly synthesized LURE1 and pollen tube attraction to be reactivated. The phenomenon in g was observed in every experiment reported in Fig. 4a (leftmost two panels). The data presented here were pooled from two independent experiments, designated to collect the numerical data presented here for discussion. i, Unpollinated but ageing ovules maintained LURE1–GFP at their filiform apparatus and thus should continue to be competent in pollen tube attraction. These data also demonstrate that NO accumulation at the filiform apparatus does not correlate with ovule age, providing further support for the notion that NO accumulates in response to pollen tube arrival. These observations were similar to those in Fig. 4a (left) and control experiments shown in Extended Data Fig. 6a–c. Scale bars, 50 μm. Arrows, filiform apparatus region.
Supplementary information
Supplementary Figure 1
Workflow for Iodoacetyl Tandem Mass Tag (iodoTMT) of MBP-LURE1 for LC-MS/MS analysis according to Thermo Scientific Manual with modifications.
Rights and permissions
About this article
Cite this article
Duan, Q., Liu, MC.J., Kita, D. et al. FERONIA controls pectin- and nitric oxide-mediated male–female interaction. Nature 579, 561–566 (2020). https://doi.org/10.1038/s41586-020-2106-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2106-2
This article is cited by
-
Near-infrared-II photoacoustic imaging and photo-triggered synergistic treatment of thrombosis via fibrin-specific homopolymer nanoparticles
Nature Communications (2023)
-
Structure and growth of plant cell walls
Nature Reviews Molecular Cell Biology (2023)
-
FERONIA coordinates plant growth and salt tolerance via the phosphorylation of phyB
Nature Plants (2023)
-
Why is FERONIA pleiotropic?
Nature Plants (2023)
-
Whole-mount RNA in situ hybridization technique in Torenia ovules
Plant Reproduction (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.