Abstract
The sympathetic nervous system innervates peripheral organs to regulate their function and maintain homeostasis, whereas target cells also produce neurotrophic factors to promote sympathetic innervation1,2. The molecular basis of this bi-directional communication remains to be fully determined. Here we use thermogenic adipose tissue from mice as a model system to show that T cells, specifically γδ T cells, have a crucial role in promoting sympathetic innervation, at least in part by driving the expression of TGFβ1 in parenchymal cells via the IL-17 receptor C (IL-17RC). Ablation of IL-17RC specifically in adipose tissue reduces expression of TGFβ1 in adipocytes, impairs local sympathetic innervation and causes obesity and other metabolic phenotypes that are consistent with defective thermogenesis; innervation can be fully rescued by restoring TGFβ1 expression. Ablating γδ Τ cells and the IL-17RC signalling pathway also impairs sympathetic innervation in other tissues such as salivary glands. These findings demonstrate coordination between T cells and parenchymal cells to regulate sympathetic innervation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Source Data for Figs. 1–3 and Extended Data Figs. 1–5 are provided with the paper. Proteomics raw data were deposited in the PRIDE archive and can be accessed via the ProteomeXchange under accession number PXD017424. RNA-seq data are available at the Gene Expression Omnibus (GEO) repository under accession number GSE144255. Any other relevant data are available from the corresponding author upon reasonable request.
References
McCorry, L. K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 71, 78 (2007).
Mattson, M. P. & Wan, R. Neurotrophic factors in autonomic nervous system plasticity and dysfunction. Neuromolecular Med. 10, 157–168 (2008).
Furlan, A. et al. Visceral motor neuron diversity delineates a cellular basis for nipple- and pilo-erection muscle control. Nat. Neurosci. 19, 1331–1340 (2016).
Morrison, S. F. Central neural control of thermoregulation and brown adipose tissue. Auton. Neurosci. 196, 14–24 (2016).
Lowell, B. B. & Spiegelman, B. M. Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 (2000).
Bachman, E. S. et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 (2002).
Daniel, H. & Derry, D. M. Criteria for differentiation of brown and white fat in the rat. Can. J. Physiol. Pharmacol. 47, 941–945 (1969).
Zeng, W. et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Fedorenko, A., Lishko, P. V. & Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 (2012).
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015).
Zeng, X. et al. Innervation of thermogenic adipose tissue via a calsyntenin 3β–S100b axis. Nature 569, 229–235 (2019).
Seale, P. et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105 (2011).
Chi, J. et al. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab. 27, 226–236 (2018).
Villarroya, F., Cereijo, R., Villarroya, J., Gavaldà-Navarro, A. & Giralt, M. Toward an understanding of how immune cells control brown and beige adipobiology. Cell Metab. 27, 954–961 (2018).
Sim, G. K., Olsson, C. & Augustin, A. Commitment and maintenance of the αβ and γδ T cell lineages. J. Immunol. 154, 5821–5831 (1995).
Papotto, P. H., Ribot, J. C. & Silva-Santos, B. IL-17+ γδ T cells as kick-starters of inflammation. Nat. Immunol. 18, 604–611 (2017).
Vantourout, P. & Hayday, A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 13, 88–100 (2013).
Cua, D. J. & Tato, C. M. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489 (2010).
Jin, C. et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell 176, 998–1013 (2019).
Ho, A. W. & Gaffen, S. L. IL-17RC: a partner in IL-17 signaling and beyond. Semin. Immunopathol. 32, 33–42 (2010).
Jin, W. & Dong, C. IL-17 cytokines in immunity and inflammation. Emerg. Microbes Infect. 2, e60 (2013).
Ouyang, W., Kolls, J. K. & Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 (2008).
Iwakura, Y., Ishigame, H., Saijo, S. & Nakae, S. Functional specialization of interleukin-17 family members. Immunity 34, 149–162 (2011).
Cheng, L. et al. Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat. Neurosci. 20, 804–814 (2017).
Starnes, T. et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J. Immunol. 167, 4137–4140 (2001).
Brionne, T. C., Tesseur, I., Masliah, E. & Wyss-Coray, T. Loss of TGF-β1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40, 1133–1145 (2003).
Krieglstein, K., Strelau, J., Schober, A., Sullivan, A. & Unsicker, K. TGF-β and the regulation of neuron survival and death. J. Physiol. Paris 96, 25–30 (2002).
Kohlgruber, A. C. et al. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol. 19, 464–474 (2018).
Sulaiman, W. & Nguyen, D. H. Transforming growth factor beta 1, a cytokine with regenerative functions. Neural Regen. Res. 11, 1549–1552 (2016).
Kumar, P. et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity 44, 659–671 (2016).
Eguchi, J. et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 13, 249–259 (2011).
Gerhart-Hines, Z. et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 503, 410–413 (2013).
Emmett, M. J. et al. Histone deacetylase 3 prepares brown adipose tissue for acute thermogenic challenge. Nature 546, 544–548 (2017).
Roark, C. L. et al. Subset-specific, uniform activation among Vγ6/Vδ1+ γδ T cells elicited by inflammation. J. Leukoc. Biol. 75, 68–75 (2004).
Elias, J. E. & Gygi, S. P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 (2007).
Huttlin, E. L. et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 (2010).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Cornwell, M. et al. VIPER: Visualization Pipeline for RNA-seq, a Snakemake workflow for efficient and complete RNA-seq analysis. BMC Bioinformatics 19, 135 (2018).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Hodges, M. R. et al. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J. Neurosci. 28, 2495–2505 (2008).
Zeng, X. et al. Lysine-specific demethylase 1 promotes brown adipose tissue thermogenesis via repressing glucocorticoid activation. Genes Dev. 30, 1822–1836 (2016).
Acknowledgements
We thank M. L. Mather, E. Maratos-Flier, R. Garrity, Z. Deng, A. Mina and D. Cabarkapa for help with CLAMS studies. We thank T. Jacks for his support and input for this project as C.J.’s postdoctoral advisor. We thank S. L. Gaffen and Y. Iwakura for sharing the Il17f −/− mice, J. K. Kolls for the Il17rc fl/fl mice, and Amgen for the Il17rc −/− and Il17ra −/− mice. We thank A. Mann, N. Asinovski and K. Hattori for the Vγ6Vδ1 transgenic mice and the 17D1 antibody. We thank C. Zhao for help with the immunofluorescence staining and confocal imaging analysis; L. Kazak and E. T. Chounchani for help with the EMG study; and R. J. Akhurst and S. Basu for sharing mice. We thank the Nikon Imaging Center at Harvard Medical School for all imaging studies; the Rodent Histology Core at Harvard Medical School for histology studies; the viral core at Children’s Hospital Boston for AAV production; and the Neurobiology Department and the Neurobiology Imaging Facility for consultation and instrument availability that supported this work. This facility is supported in part by the Neural Imaging Center as part of an NINDS P30 Core Center grant NS072030. We thank Z. Herbert, A. Caruso and members of the Molecular Biology Core Facilities at the Dana-Farber Cancer Institute for RNA-seq analysis. We thank the Flow Cytometry Core Facilities at the Swanson Biotechnology Center at MIT and the Dana-Farber Cancer Institute. We thank Y. Chen, D. Bogoslavsk, J. Szpyt and all members of the Spiegelman and Mathis laboratories for help and input in this project. B.H. is a Cancer Research Institute/Leonard Kahn Foundation Fellow. C.J. is supported by a K99 Award (CA226400) from the National Cancer Institute (NCI). This work was supported by grants to B.M.S. from the NIH DK 31405 and from the JPB Foundation.
Author information
Authors and Affiliations
Contributions
B.H. and B.M.S. conceived and directed the study. B.H., C.J., X.Z., J.M.R., Z.Y., B.B.L., D.M. and B.M.S designed and performed experiments and analysed the data. M.P.J. performed mass spectrometry analysis. J.R., Z.Y. and B.B.L. helped with chemogenetic experiments. B.N.D. and A.S.B. helped with CLAMS studies. B.H., C.J., X.Z. and B.M.S. wrote the manuscript with comments from all authors. All authors provided input and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Bruno Silva-Santos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 γδ T cells promote adaptive thermogenesis.
a, Reduced oxygen consumption (VO2) of Tcrd −/− mice during cold exposure measured by indirect calorimetry (n = 9 mice). b, Increased lipid accumulation in the BAT of Tcrd −/− mice as measured by haematoxylin and eosin (H&E) staining. Scale bars, 100 μm. c, No significant differences in food intake (left) or physical movement (right) in wild-type and Tcrd −/− mice, as measured by indirect calorimetry (n = 9 mice). Xtotal count, total number of x-axis infrared-beam breaks count. d, No significant change in electromyography (EMG) root mean square (RMS) muscle activity in wild-type and Tcrd −/− mice during cold exposure (n = 4 mice). e, A significant population of γδ T cells in BAT are Vγ6Vδ1+ (n = 6 mice). f, Flow cytometry analysis of γδ Τ cells in BAT. g, Cold exposure (6 h) upregulates Il17f mRNA expression in BAT (n = 5 mice). RT, room temperature. Cyclo, cyclophilin B (also known as Ppib). Data are representative of at least two or three independent experiments. Data are mean ± s.e.m. P values were determined by two-way ANOVA (a, c) or unpaired Student’s two-sided t-test (d, g).
Extended Data Fig. 2 IL-17RC deficiency predisposes mice to cold sensitivity and obesity.
a, b, Il17rc mRNA expression shown by translating ribosomal affinity purification (TRAP) from adiponectin-positive (n = 3 mice) (a) and UCP1-positive (b) cells (BAT, iWAT n = 3 mice; eWAT n = 2 mice). eWAT, epididymal white adipose tissue; FPKM, fragments per kilobase of transcript per million mapped reads; iWAT, inguinal white adipose tissue. c, Il17ra−/− mice are not sensitive to cold exposure (WT n = 13, Il17ra−/− n = 10 mice). d, Increased lipid accumulation in the BAT of AdIl17rc-KO mice as determined by H&E histology. Scale bars, 100 μm. e, Ucp1-cre;Il17rcfl/fl mice are sensitive to acute cold exposure (WT n = 7, Ucp1-cre;Il17rcfl/fl n = 6 mice). f, AdIl17rc-KO;Vγ6Vδ1 transgenic mice are sensitive to acute cold exposure (WT n = 8; AdIl17rc-KO;Vγ6Vδ1, AdIl17rc-KO n = 5 mice). g, Reduced oxygen consumption (VO2) induced by a high-fat diet (HFD) in AdIl17rc-KO mice as measured by indirect calorimetry (2 weeks after the start of high-fat diet feeding) (n = 8 mice). h, i, No significant differences in physical movement (h) or food intake (i) in wild-type and AdIl17rc-KO mice (n = 8). j, k, H&E histology of iWAT (j) and eWAT (k) of high-fat-diet-treated wild-type and AdIl17rc-KO mice (n = 8 mice). Scale bars, 100 μm. Data are mean ± s.e.m. and representative of at least two or three independent experiments. P values were determined by unpaired Student’s two-sided t-test (h, i) or two-way ANOVA (c, e–g).
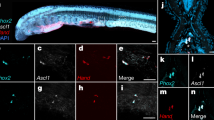
Extended Data Fig. 3 IL-17RC signalling deficiency impairs sympathetic innervation in adipose and multiple tissue.
a, Reduced sympathetic innervation of BAT in Rag2 −/− mice as measured by TH (green) and TUBB3 (yellow) immunostaining (n = 6 mice). b, Reduced sympathetic innervation of BAT in Il17f −/− but not Il17a −/− mice by TH (green) and TUBB3 (yellow) immunostaining (WT n = 6, Il17f −/− n = 5, Il17a −/− n = 3 mice). c, Reduced sympathetic innervation of iWAT in AdIl17rc-KO mice by TH immunostaining (n = 6 mice). d, FOS immunoreactivity in response to CNO administration. e, Forced expression of S100B by AAV-S100B rescues the cold sensitivity in AdIl17rc-KO mice (n = 5 mice). f, Decreased lipid accumulation in the AAV-S100B-treated BAT, as measured by H&E histology. g, Forced expression of S100B by AAV-S100B increases adipose innervation in AdIl17rc-KO mice by TH (green) and TUBB3 (yellow) immunostaining (n = 6 mice). h, Forced expression of CLSTN3β by AAV-DIO-CLSTN3β rescues the cold sensitivity in AdIl17rc-KO mice (n = 5 mice). i, Decreased lipid accumulation in the AAV-DIO-CLSTN3β-treated BAT as measured by H&E histology. j, Forced expression of CLSTN3β by AAV-DIO-CLSTN3β increases adipose innervation in AdIl17rc-KO mice by TH (green) and TUBB3 (yellow) immunostaining (n = 6 mice). k, Reduced sympathetic innervation of salivary glands in Il17rc −/− mice by TH (green) and TUBB3 (yellow) immunostaining of WT and Il17rc KO SG (n = 6 mice). l, Reduced sympathetic innervation of salivary glands in Tcrd −/−, Il17f −/− but not Il17a −/− mice by TH (green) and TUBB3 (yellow) staining (WT n = 6, Tcrd −/− n = 6, Il17f −/− n = 6, Il17a −/− n = 3 mice). m, Reduced neuronal innervation of salivary glands in Rag2 −/− mice by TH (green) and TUBB3 (yellow) immunostaining. (n = 4 mice). n, No significant difference in sympathetic innervation of salivary glands in AdIl17rc-KO mice by TH (green) and TUBB3 (yellow) immunostaining (n = 3 mice). o, Reduced sympathetic innervation of bronchi in Rag2 −/− and Tcrd −/− mice by TH and TUBB3 staining (WT n = 8, Rag2 −/− n = 3, Tcrd −/− n = 4, Il17rc −/− n = 3 mice). Data are mean ± s.e.m. and are representative of at least two or three independent experiments. P values were determined by one-way ANOVA with Bonferroni’s multiple comparisons test (b, l), unpaired Student’s two-sided t-test (a, g, j, k, m, n) or two-way ANOVA (e, h). Scale bars, 50 μm (a, b, g, j–o), 100 μm (c, d, f, i).
Extended Data Fig. 4 Reduced Tgfb1 and collagen genes in AdIl17rc-KO mice.
a, Pathway enrichment analysis of genes downregulated both at the RNA and the protein level in BAT from AdIl17rc-KO mice compared with littermate controls. b, Reduced mRNA expression of Tgfb1, Col1a1, Col3a1, Col5a1 and Ncor2 in the BAT of AdIl17rc-KO mice (n = 3 mice). Data are representative of at least two or three independent experiments. c–e, Reduced Tgfb1 mRNA expression in BAT, salivary glands (SG) and lungs of Il17rc −/−, Tcrd −/−, Il17f −/− and Rag2 −/− mice (BAT: WT n = 7, Il17rc −/− n = 6; WT n = 5, Tcrd −/− n = 6, Il17f −/− n = 6; WT, Rag2 −/− n = 4 mice; SG: WT n = 4, Il17rc −/− n = 3; WT, Tcrd −/−, Il17f −/− n = 3; WT, Rag2 −/− n = 3 mice; lung: WT n = 5, Il17rc −/− n = 4; WT n = 6, Tcrd −/− n = 4, Il17f −/− n = 6; WT, Rag2 −/− n = 6 mice). Cyclo, cyclophilin B (also known as Ppib). Data are mean ± s.e.m. P values or FDR q values determined by unpaired Student’s two-sided t-test (b, c, e), as described in the Methods (a), or one-way ANOVA with Bonferroni’s multiple comparisons test (d).
Extended Data Fig. 5 Blocking TGFβ sensitizes mice to acute cold exposure.
a, Decreased lipid accumulation in BAT from AAV-DIO-TGFβ1-treated mice as determined by H&E histology. Data are representative of two independent experiments. b, Wild-type mice treated with TGFβ-neutralizing antibody (Ab)) for 3 weeks are sensitive to cold exposure(n = 6 mice). c, Wild-type mice treated with TGFβ inhibitor (sb431542) for 3 weeks are sensitive to cold exposure (n = 8). d, e, Forced expression of TGFβ1-AAV increases the TH (d) and TUBB3 (e) immunostaining in the salivary glands from Il17rc −/− mice (n = 6 mice). f, g, Forced expression of AAV-TGFβ1 increases TH (f) and TUBB3 (g) immunostaining in the salivary glands from Rag2 −/− mice (n = 6 mice). Scale bars, 50 μm. Data are mean ± s.e.m. and representative of two or three independent experiments. P values were determined by two-way ANOVA (b, c) or unpaired Student’s two-sided t-test (d–g).
Supplementary information
Rights and permissions
About this article
Cite this article
Hu, B., Jin, C., Zeng, X. et al. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614 (2020). https://doi.org/10.1038/s41586-020-2028-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2028-z
This article is cited by
-
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2024)
-
Somatosensory cortex and central amygdala regulate neuropathic pain-mediated peripheral immune response via vagal projections to the spleen
Nature Neuroscience (2024)
-
IL-17 signalling is critical for controlling subcutaneous adipose tissue dynamics and parasite burden during chronic murine Trypanosoma brucei infection
Nature Communications (2023)
-
γδ T cells control murine skin inflammation and subcutaneous adipose wasting during chronic Trypanosoma brucei infection
Nature Communications (2023)
-
Senescent immune cells accumulation promotes brown adipose tissue dysfunction during aging
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.