Abstract
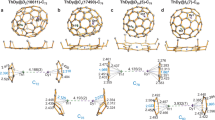
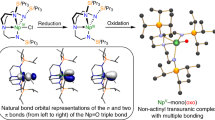
Aromaticity and antiaromaticity, as defined by Hückel’s rule, are key ideas in organic chemistry, and are both exemplified in biphenylene1,2,3—a molecule that consists of two benzene rings joined by a four-membered ring at its core. Biphenylene analogues in which one of the benzene rings has been replaced by a different (4n + 2) π-electron system have so far been associated only with organic compounds4,5. In addition, efforts to prepare a zirconabiphenylene compound resulted in the isolation of a bis(alkyne) zirconocene complex instead6. Here we report the synthesis and characterization of, to our knowledge, the first 2-metallabiphenylene compounds. Single-crystal X-ray diffraction studies reveal that these complexes have nearly planar, 11-membered metallatricycles with metrical parameters that compare well with those reported for biphenylene. Nuclear magnetic resonance spectroscopy, in addition to nucleus-independent chemical shift calculations, provides evidence that these complexes contain an antiaromatic cyclobutadiene ring and an aromatic benzene ring. Furthermore, spectroscopic evidence, Kohn–Sham molecular orbital compositions and natural bond orbital calculations suggest covalency and delocalization of the uranium f2 electrons with the carbon-containing ligand.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available upon request from J.L.K. (synthetic and spectroscopic data) or L.G. (computational data). Crystallographic data of 4 and 5 have been uploaded to the Cambridge Crystallographic Data Centre under CCDC numbers 1526648 and 1526649.
References
Karadakov, P. B., Hearnshaw, P. & Horner, K. E. Magnetic shielding, aromaticity, antiaromaticity, and bonding in the low-lying electronic states of benzene and cyclobutadiene. J. Org. Chem. 81, 11346–11352 (2016).
Balaban, A. T. & Vollhardt, K. P. C. Heliphenes and related structures. Open Org. Chem. J. 5, 117–126 (2011).
Vollhardt, K. P. C. & Mohler, D. L. The phenylenes: synthesis, properties, and reactivity. Adv. Strain Org. Chem. 5, 121–160 (1996).
Lothrop, W. C. Biphenylene. J. Am. Chem. Soc. 63, 1187–1191 (1941).
Garratt, P. J. & Vollhardt, K. P. C. Benzo[3,4]cyclobuta[1,2-c]thiophen (2-thianorbiphenylene). J. Chem. Soc. D., Chem. Commun. 109 (1970).
Warner, B. P., Davis, W. M. & Buchwald, S. L. Synthesis and X-ray structure of a zirconocene complex of two alkynes. J. Am. Chem. Soc. 116, 5471–5472 (1994).
Boese, R., Blaser, D. & Latz, R. Redetermination of biphenylene at 130 K. Acta Crystallogr. C C55, IUC9900067 (1999).
Fawcett, J. K. & Trotter, J. A Refinement of the structure of biphenylene. Acta Crystallogr. 20, 87–93 (1966).
Fang, B., Hou, G., Zi, G., Fang, D.-C. & Walter, M. D. A thorium metallacyclopentadiene complex: a combined experimental and computational study. Dalton Trans. 44, 7927–7934 (2015).
Pagano, J. K., Dorhout, J. M., Waterman, R., Czerwinski, K. R. & Kiplinger, J. L. Phenylsilane as a safe, versatile alternative to hydrogen for the synthesis of actinide hydrides. Chem. Commun. 51, 17379–17381 (2015).
Evans, W. J., Kozimor, S. A. & Ziller, J. W. [(C5Me5)2U][(μ-Ph)2BPh2] as a four electron reductant. Chem. Commun. 37,4681–4683 (2005).
Pagano, J. K. et al. Tuning the oxidation state, nuclearity, and chemistry of uranium hydrides with phenylsilane and temperature: the case of the classic uranium(III) hydride complex [(C5Me5)2U(μ-H)]2. Organometallics 35, 617–620 (2016).
Bettinger, H. F., Schleyer, P. R. & Schaefer, H. F., III. Tetraphenyldihydrocyclobutaarenes—what causes the extremely long 1.72 Å C–C single bond? Chem. Commun. 769–770 (1998).
Toda, F. Naphthocyclobutenes and benzodicyclobutadienes: synthesis in the solid state and anomalies in the bond lengths. Eur. J. Org. Chem. 2000, 1377–1386 (2000).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Jantunen, K. C. et al. Thorium(IV) and uranium(IV) ketimide complexes prepared by nitrile insertion into actinide–alkyl and –aryl bonds. Organometallics 23, 4682–4692 (2004).
Lu, E. et al. Synthesis, characterization, and reactivity of a uranium(VI) carbene imido oxo complex. Angew. Chem. Int. Ed. 53, 6696–6700 (2014).
Schleyer, P. R., Maerker, C., Dransfeld, A., Jiao, H. & van Eikema Hommes, N. J. Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J. Am. Chem. Soc. 118, 6317–6318 (1996).
Merino, G., Heine, T. & Seifert, G. The induced magnetic field in cyclic molecules. Chem. Eur. J. 10, 4367–4371 (2004).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Andersson, K., Malmqvist, P. Å. & Roos, B. O. Second-order perturbation theory with a complete active space self-consistent field reference function. J. Chem. Phys. 96, 1218–1226 (1992).
Roos, B. O., Taylor, P. R. & Sigbahn, P. E. M. A complete active space SCF method (CASSCF) using a density matrix formulated super-CI approach. Chem. Phys. 48, 157–173 (1980).
Heit, Y. N., Gendron, F. & Autschbach, J. Calculation of dipole-forbidden 5f absorption spectra of uranium(V) hexa-halide complexes. J. Phys. Chem. Lett. 9, 887–894 (2018).
Lever, A. B. P. (ed.) Inorganic Electronic Spectroscopy 2nd edn, Ch. 4, 161–202 (Elsevier, 1999).
Pagano, J. K. et al. Synthesis and characterization of a new and electronically unusual uranium metallacyclocumulene, (C5Me5)2U(η4-1,2,3,4-PhC4Ph). J. Organomet. Chem. 829, 79–84 (2017).
Morris, D. E., Da Re, R. E., Jantunen, K. C., Castro-Rodriguez, I. & Kiplinger, J. L. Trends in electronic structure and redox energetics for early-actinide pentamethylcyclopentadienyl complexes. Organometallics 23, 5142–5153 (2004).
Cantat, T., Scott, B. L., Morris, D. E. & Kiplinger, J. L. What a difference a 5f element makes: trivalent and tetravalent uranium halide complexes supported by one and two bis[2-(diisopropylphosphino)-4-methylphenyl]amido (PNP) ligands. Inorg. Chem. 48, 2114–2127 (2009).
Schelter, E. J. et al. Actinide redox-active ligand complexes: reversible intramolecular electron-transfer in U(dpp-BIAN)2/U(dpp-BIAN)2(THF). Inorg. Chem. 49, 924–933 (2010).
Schelter, E. J. et al. Systematic studies of early actinide complexes: uranium(IV) fluoroketimides. Inorg. Chem. 46, 7477–7488 (2007).
Cantat, T. et al. Evidence for the involvement of 5f orbitals in the bonding and reactivity of organometallic actinide compounds: thorium(IV) and uranium(IV) bis(hydrazonato) complexes. J. Am. Chem. Soc. 130, 17537–17551 (2008).
Da Re, R. E., Jantunen, K. C., Golden, J. T., Kiplinger, J. L. & Morris, D. E. Molecular spectroscopy of uranium(IV) bis(ketimido) complexes. Rare observation of resonance-enhanced Raman scattering from organoactinide complexes and evidence for broken-symmetry excited states. J. Am. Chem. Soc. 127, 682–689 (2005).
Minasian, S. G. et al. New evidence for 5f covalency in actinocenes determined from carbon K-edge XAS and electronic structure theory. Chem. Sci. 5, 351–359 (2014).
Peterson, P. W. et al. Orbital crossings activated through electron injection: opening communication between orthogonal orbitals in anionic C1–C5 cyclizations of enediynes. J. Am. Chem. Soc. 138, 15617–15628 (2016).
Fagan, P. J., Manriquez, J. M., Maatta, E. A., Seyam, A. M. & Marks, T. J. Synthesis and properties of bis(pentamethylcyclopentadienyl) actinide hydrocarbyls and hydrides. A new class of highly reactive f-element organometallic compounds. J. Am. Chem. Soc. 103, 6650–6667 (1981).
APEX2 v.2014.7-1 (Bruker AXS, 2014).
SAINT v.8.34A (Bruker AXS, 2013).
SADABS v.2014/3 (Bruker AXS, 2013).
SHELXTL v.6.14 (Bruker AXS, 2000).
Gaussian 09, revision E.01 (Gaussian Inc., 2009).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Su, J. et al. Energy-degeneracy-driven covalency in actinide bonding. J. Am. Chem. Soc. 140, 17977–17984 (2018).
Vukovic, S., Hay, B. P. & Bryantsev, V. S. Predicting stability constants for uranyl complexes using density functional theory. Inorg. Chem. 54, 3995–4001 (2015).
Hu, H.-S., Wei, F., Wang, X., Andrews, L. & Li, J. Actinide–silicon multiradical bonding: infrared spectra and electronic structures of the Si(μ-X)AnF3 (An = Th, U; X = H, F) molecules. J. Am. Chem. Soc. 136, 1427–1437 (2014).
Safi, S. et al. Osteopontin: a uranium phosphorylated binding-site characterization. Chem. Eur. J. 19, 11261–11269 (2013).
Minasian, S. G. et al. Determining relative f and d orbital contributions to M–Cl covalency in MCl6 2– (M = Ti, Zr, Hf, U) and UOCl5 – using Cl K-edge X-ray absorption spectroscopy and time-dependent density functional theory. J. Am. Chem. Soc. 134, 5586–5597 (2012).
Tsushima, S., Brendler, V. & Fahmy, K. Aqueous coordination chemistry and photochemistry of uranyl (VI) oxalate revisited: a density functional theory study. Dalton Trans. 39, 10953–10958 (2010).
Kubicki, J. D., Halada, G. P., Jha, P. & Phillips, B. L. Quantum mechanical calculation of aqueous uranium complexes: carbonate, phosphate, organic and biomolecular species. Chem. Cent. J. 3, 10 (2009).
Graves, C. R. et al. Organometallic uranium(V)−imido halide complexes: from synthesis to electronic structure and bonding. J. Am. Chem. Soc. 130, 5272–5285 (2008).
Gutowski, K. E. et al. Interactions of 1-methylimidazole with UO2(CH3CO2)2 and UO2(NO3)2: structural, spectroscopic, and theoretical evidence for imidazole binding to the uranyl ion. J. Am. Chem. Soc. 129, 526–536 (2007).
Cao, X., Dolg, M. & Stoll, H. Valence basis sets for relativistic energy-consistent small-core actinide pseudopotentials. J. Chem. Phys. 118, 487–496 (2003).
Hehre, W. J., Ditchfield, R. & Pople, J. A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56, 2257–2261 (1972).
Krishnan, R., Binkley, J. S., Seeger, R. & Pople, J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Yanai, T., Tew, D. P. & Handy, N. C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Zhao, Y. & Truhlar, D. G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 125, 194101 (2006).
Wolinski, K., Hinton, J. F. & Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 112, 8251–8260 (1990).
Andersson, K., Malmqvist, P. A., Roos, B. O., Sadlej, A. J. & Wolinski, K. Second-order perturbation theory with a CASSCF reference function. J. Phys. Chem. 94, 5483–5488 (1990).
Aquilante, F. et al. MOLCAS 8: new capabilities for multiconfigurational quantum chemical calculations across the periodic table. J. Comput. Chem. 37, 506–541 (2016).
Roos, B. O., Lindh, R., Malmqvist, P. Å., Veryazov, V. & Widmark, P. O. Main group atoms and dimers studied with a new relativistic ano basis set. J. Phys. Chem. A 108, 2851–2858 (2004).
Roos, B. O., Lindh, R., Malmqvist, P. Å., Veryazov, V. & Widmark, P. O. New relativistic ano basis sets for actinide atoms. Chem. Phys. Lett. 409, 295–299 (2005).
Douglas M. & Kroll, N. M. Quantum electrodynamical corrections to fine-structure of helium. Ann. Phys. 82, 89–155 (1974).
Hess, B. A. Applicability of the no-pair equation with free-particle projection operators to atomic and molecular-structure calculations. Phys. Rev. A 32, 756–763 (1985).
Aquilante, F., Pedersen, T. B. & Lindh, R. Unbiased auxiliary basis sets for accurate two-electron integral approximations. J. Chem. Phys. 127, 114107 (2007).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Acknowledgements
For financial support of this work, we acknowledge the US Department of Energy (DOE) through the Los Alamos National Laboratory (LANL) Laboratory Directed Research and Development Program; the LANL G. T. Seaborg Institute for Transactinium Science (Postdoctoral Fellowships to J.K.P., K.A.E. and S.K.C.); the Office of Workforce Development for Teachers and Scientists, Office of Science Graduate Student Research (SCGSR) program (GRA Fellowship to J.K.P.); and the Office of Basic Energy Sciences, Heavy Element Chemistry program (to J.L.K., P.Y. and B.L.S., for materials and supplies). We thank R. Michalczyk and L. A. Silks (LANL) for assistance with two-dimensional NMR experiments, and J. Thompson and P. Rosa (LANL) for assistance in collecting magnetometry data. This work was in part funded by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the US DOE through grant USDOE/DESC002183 (to J.X. and L.G.). We also acknowledge the US National Science Foundation (grants CHE-1265608 and CHE-1565658 to R. Waterman). The SCGSR program is administered by the Oak Ridge Institute for Science and Education for the DOE (contract DE-AC05-06OR23100). LANL is operated by Los Alamos National Security, LLC, for the National Nuclear Security Administration of the US DOE (contract DE-AC52-06NA25396).
Author information
Authors and Affiliations
Contributions
J.K.P. synthesized and fully characterized compounds 4 and 5, including collecting and solving single-crystal X-ray data. J.X. performed computational calculations and wrote the calculation part of the manuscript. J.X. used the Minnesota Supercomputing Institute (University of Minnesota) for computational resources. K.A.E. provided the alternative synthesis of compound 5 and spectroscopically characterized compound 1. S.K.C. performed two-dimensional NMR experiments on compounds 4 and 5 and interpreted the data. D.E.M. interpreted electronic spectra and wrote that section of the manuscript. B.L.S. maintained the X-ray facility and provided assistance with solutions. R. Wu synthesized 1,2-bis(phenylethynyl)benzene. P.Y. assisted with time-dependent DFT calculations on compounds 4 and 5. J.K.P., J.X., K.A.E. and J.L.K. wrote the initial manuscript. J.L.K. and R. Waterman supervised the synthetic research. D.E.M. and J.L.K. supervised the spectroscopic characterization. L.G. supervised the computational calculations. J.L.K. initiated the research. All authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Joy Farnaby and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
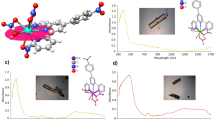
Extended Data Fig. 1 NIR spectroscopy as a covalency indicator.
NIR spectra of U(iv)-containing compounds 5 and 7 collected in toluene at 298 K. Band energies and relative intensities are strongly correlated in these spectra, but the absolute intensities in the bands for 5 are around two to four times those in the bands for 7, which suggests a greater degree of U–C covalency.
Extended Data Fig. 2 NICS profiles to assess aromaticity/antiaromaticity.
A, NICS and Bindz values were computed in the axis perpendicular to the four-membered ring and six-membered ring planes of compounds 4 and 5. The points at which the shielding tensors were calculated are represented here by small blue spheres; the points pass through the corresponding non-mass-weighted centre of each ring. B–D, NICS and Bindz profiles of the four-membered ring and six-membered ring for compounds 4 (B; An = Th), 5 (C; An = U), and cyclobutadiene and benzene (D). The PBE0/6-31G(d,p)&SDD level of theory was used. R is the distance from the centre of each ring along the axis, in Å.
Extended Data Fig. 3 NICS profiles of classic compounds to assess aromaticity/antiaromaticity.
A–D, NICS and Bindz profiles of the four-membered ring and six-membered ring for biphenylene (1) (A), 2-thianorbiphenylene (2) (B), 2-thianorbiphenylene sulfone (3) (C), and benzo[3,4]cyclobuta[1,2]furan (D).
Extended Data Fig. 4 Calculated Kohn–Sham molecular orbitals of compound 5.
Molecular orbitals for triplet (C5Me5)2U(2,5-Ph2-cyclopenta[3,4]cyclobuta[1,2]benzene) (5-triplet) as computed with PBE0/631G(d,p)&SDD. An isocontour value of 0.04 was used. The left column shows the α-spin molecular orbitals and the right column shows the β-spin molecular orbitals. For both spins, the orbitals are labelled according to their energetic order among those of the same spin. Orbitals are paired here with the closest corresponding orbital of the opposite spin. The positive and negative phases of the Kohn–Sham molecular orbitals are in red and blue, respectively.
Extended Data Fig. 5 Calculated active molecular orbitals and natural bond orbitals.
A–F, Active molecular orbitals for the CAS(6,6) model of 5a-triplet (An = U) in its triplet ground spin state, with the occupation number given in parentheses. The positive and negative phases of the active molecular orbitals are in blue and white. G–I, Representative natural bond orbitals for 5-triplet (An = U) in the triplet state. The positive and negative phases of the natural bond orbitals are in red and yellow. U, green; C, silver. H atoms are omitted for clarity.
Supplementary information
Supplementary Information
This file includes Experimental SI, Figures S1–S9, Table S1, Computational SI, Figures S10–S20, Tables S2–S18 and Calculated Structures Coordinates.
Rights and permissions
About this article
Cite this article
Pagano, J.K., Xie, J., Erickson, K.A. et al. Actinide 2-metallabiphenylenes that satisfy Hückel’s rule. Nature 578, 563–567 (2020). https://doi.org/10.1038/s41586-020-2004-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2004-7
This article is cited by
-
Ring contraction of metallacyclobutadiene to metallacyclopropene driven by π- and σ-aromaticity relay
Nature Synthesis (2022)
-
A tris-spiro metalla-aromatic system featuring Craig-Möbius aromaticity
Nature Communications (2021)
-
Isolation and characterization of a californium metallocene
Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.